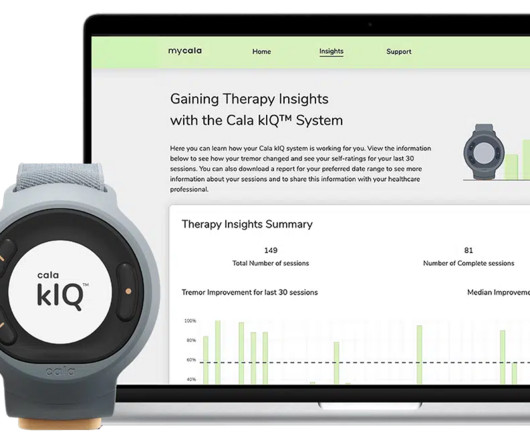
Cala® Launches The Cala kIQ™ System, Offering Meaningful Tremor Relief for Patients With Essential Tremor and Now Parkinson’s Disease
Legacy MEDSearch
JUNE 15, 2023
“At Cala, we’ve always been committed to expanding patient access and accelerating innovation in TAPS therapy for indications beyond essential tremor,” said Renee Ryan, CEO at Cala. “By By expanding into Parkinson’s disease, we are now able to bring this powerful treatment to additional patients who suffer from action hand tremors.”











Let's personalize your content