Ipsen concludes purchase of Albireo Pharma
Pharmaceutical Technology
MARCH 3, 2023
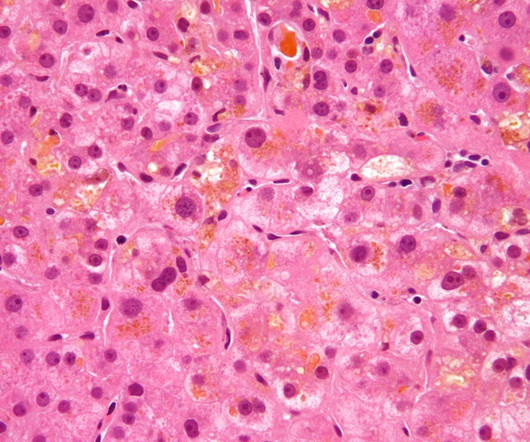
Albireo’s pipeline includes its lead asset, Bylvay (odevixibat), a potent, once-a-day, oral, non-systemic, ileal bile acid transport inhibitor (IBATi). In 2021, it secured regulatory approvals in the US to treat pruritus in progressive familial intrahepatic cholestasis (PFIC) in patients aged three months and above.












Let's personalize your content