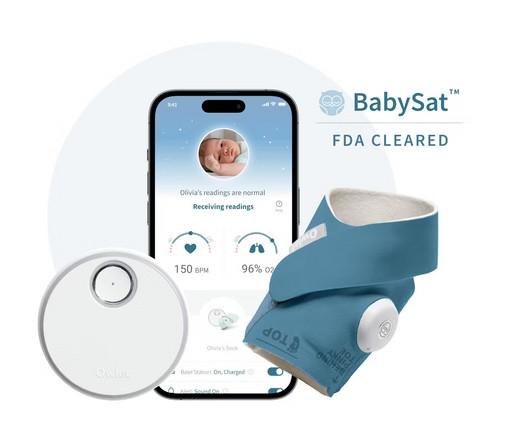
Owlet Announces FDA-Clearance of the First Prescription Pulse Oximetry Sock for Infants
Legacy MEDSearch
JUNE 20, 2023
Food and Drug Administration (“FDA”) of BabySat , the first medical pulse-oximetry device featuring its advanced, wire-free sock design. Innovation in the baby care space matters because some of the largest issues facing caregivers and healthcare providers have yet to be solved. Are you hiring?









Let's personalize your content