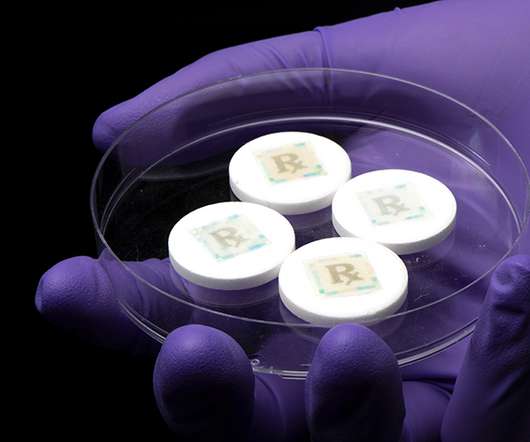
Securing every dose with an edible security technology for safe medicines
European Pharmaceutical Review
AUGUST 23, 2022
The prevalence of fake drugs is a continually growing problem worldwide. Fake drugs can be categorised as substandard, falsified, counterfeit and diverted drugs, and the World Health Organization (WHO) broadly defines a counterfeit medicine as “one which is deliberately and fraudulently mislabelled with respect to identity and/or source.”









Let's personalize your content