US FDA grants orphan drug status for XORTX’s oxypurinol
Pharmaceutical Technology
APRIL 24, 2023
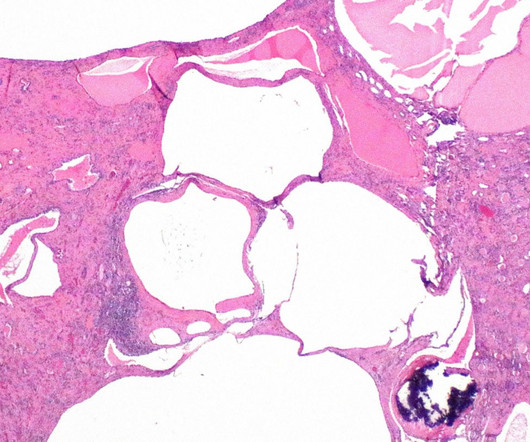
The US Food and Drug Administration (FDA) has granted orphan drug designation (ODD) to XORTX Therapeutics’ oxypurinol to treat autosomal dominant polycystic kidney disease (ADPKD) patients. We look forward to our upcoming meeting with the FDA on 1 May 2023 to discuss our planned Phase III clinical programme for XORLO.”













Let's personalize your content