Can the FDA keep the momentum going for rare disease drug approvals?
Pharmaceutical Technology
JANUARY 22, 2023
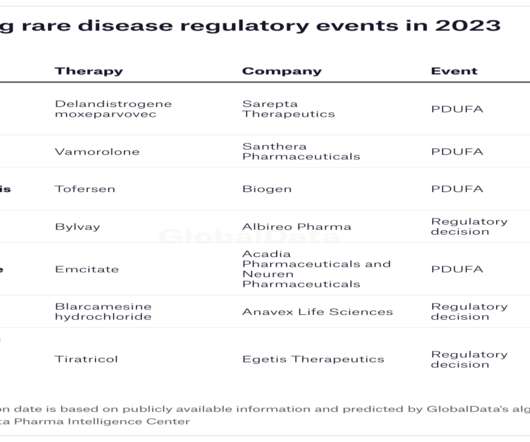
In 2022, the FDA approved only 37 new medicines, an underwhelming number compared to 98 in 2018. However, while only around 34% of the approvals in 2018 were for orphan drugs, 54% new approvals in 2022 were for drugs to treat rare diseases. The company plans to submit an MAA to the EMA in H1 this year.










Let's personalize your content