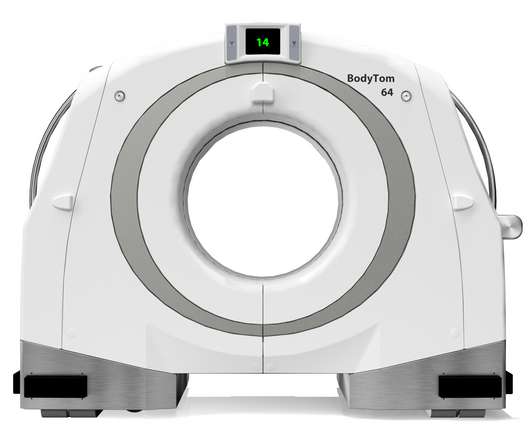
NeuroLogica Announces FDA 510(k) Clearance of BodyTom® 64
Legacy MEDSearch
NOVEMBER 28, 2022
Food and Drug Administration for commercial use in the United States. Director of Global Sales and Marketing of NeuroLogica. Trauma/ER: The BodyTom 64’s unique combination of internal lead shielding and battery operation allows any standard trauma bay to be transformed into an advanced CT imaging suite.










Let's personalize your content