Elevating pharmaceutical manufacturing processes with real-time insights
European Pharmaceutical Review
DECEMBER 4, 2023
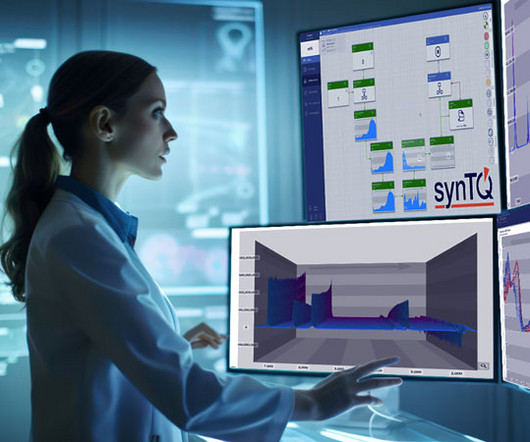
To address these issues, companies should adopt more advanced production strategies such as continuous flow chemistry, as they can shorten manufacturing processes, increase productivity and throughput. The result is efficient, cost-effective and high-quality (bio)pharmaceutical production.









Let's personalize your content