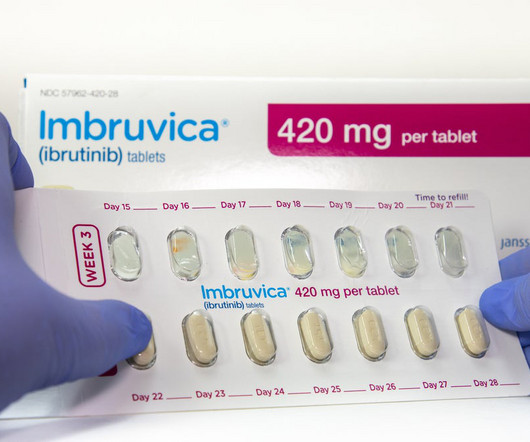
NICE recommends AbbVie’s/J&J’s Imbruvica for combo use in untreated CLL
Pharmaceutical Technology
APRIL 24, 2023
The combination therapy also caused fewer side effects, with heart problems and hypertension as the most common ones, based on the final draft guidance. While Imbruvica maintained a previously strong position on the market, competition has chipped away at the inhibitor’s market share. billion in 2029.








Let's personalize your content