Preventing fungal contamination in pharmaceuticals
European Pharmaceutical Review
MARCH 11, 2024
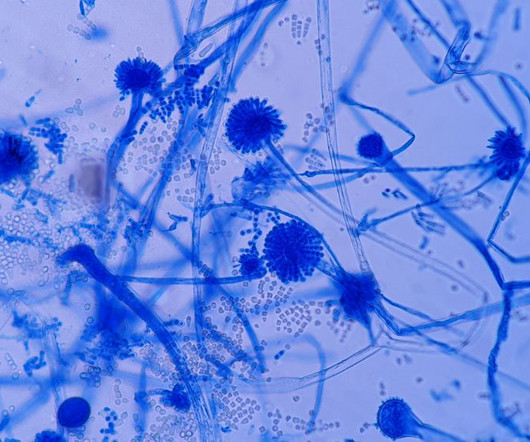
Additionally, this can be caused by “poor handling, repackaging, and nonadherence to good manufacturing practices [GMP] during dispensing and packaging have resulted in the microbiological contamination of non-sterile pharmaceutical products.” One case of drug contamination from 2021 was highlighted in the paper. Ahmed et al.







Let's personalize your content