How NLP Helps Life Sciences Companies Unlock Insights from Drug Labels
PM360
NOVEMBER 29, 2022
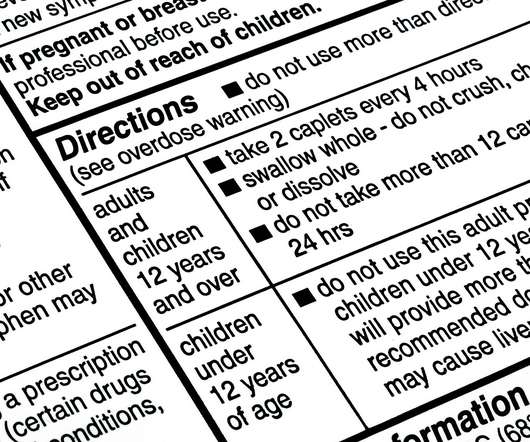
When developing content for new drug labels, life sciences companies’ regulatory teams face challenges in gathering intelligence to better understand competitive factors, market dynamics, and effective approval strategies. Food and Drug Administration (FDA) or European Medicines Agency (EMA).








Let's personalize your content