In the dynamic realm of healthcare, ensuring drug safety stands as an absolute priority for life sciences companies. To understand more about the intricate landscape of Pharmacovigilance (PV), Indegene embarked on a journey and delved deep into current practices, unveiling the challenges that PV professionals are determined to conquer, exploring how diverse organizations harness the power of technology, and catching a glimpse of the future.
The latest Pharmacovigilance Industry Survey Report for 2023 encapsulates insights from 100 seasoned PV Professionals across the USA and Europe, vividly portraying the industry's current state and future trajectory. Here are the key findings that define this insightful report:
- Priorities of PV Teams: Compliance, quality, and operational efficiency are the primary focus areas in PV. This balanced approach reflects a strong commitment to regulatory compliance, patient safety, and process optimization across the industry.
- Transformation Ahead: More than a quarter (29%) of the respondents see "Adverse Event (AE) Monitoring and Intake" as a prime candidate for transformation within the next 12 months.
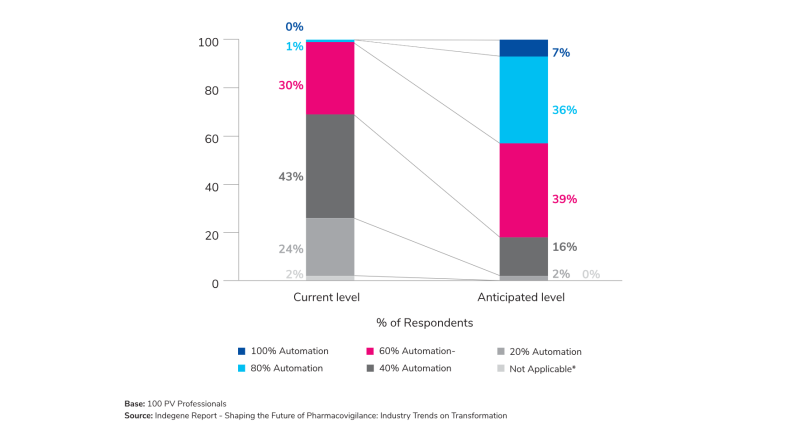
- Automation on the Rise: ~25% of the survey respondents reported their organizations are currently operating with 20% automation in Case Processing. However, they anticipated a substantial shift of Case Processing automation toward 60% and above in the next 12 months, displaying a clear upward trend toward automation.
- AI/ML Gap: The survey reveals that only 5% of organizations have used AI/ML extensively for AE Monitoring and Intake, whereas approximately 18% of respondents still rely on basic tools. On average, 47% rely on automation without AI/ML for Case Processing, Aggregate Reporting, and Signal and Risk Management. It underscores the need for improvement in technology adoption maturity levels across PV processes.
- Outsourcing Dynamics: Currently, the percentage of organizations outsourcing more than 60% of their operations is at 21% for Signal and Risk Management, 26% for AE Monitoring and Case Intake, and 40% for Case Processing. The trend indicates a shift towards higher outsourcing levels in the coming year. Organizations seek more flexibility, alignment with long-term visions, and innovation from their vendors.
One of the most striking revelations from the report is the accelerating shift towards automation in PV processes. The statistics paint a vivid picture: a surge in the adoption of advanced technologies like AI and machine learning, poised to redefine how adverse events are monitored and safety signals detected. Real-time alert mechanisms and AI-driven algorithms are slated to become cornerstones for accuracy, speed, and better patient outcomes.
Furthermore, the report sheds light on the pivotal role of strategic collaborations with PV service providers. While vendors exhibit excellence in specific areas like resource expertise and compliance, the report highlights the untapped potential for innovation and alignment with long-term strategic goals. It's a call for forging stronger partnerships, fostering innovation, and aligning services with the strategic visions of life sciences companies.
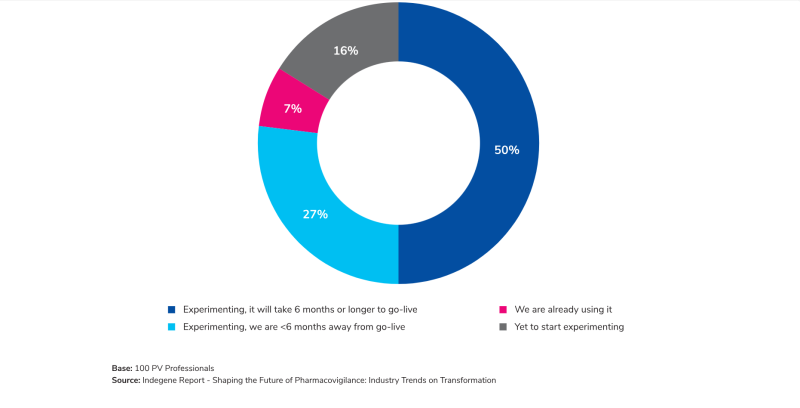
Yet, the most captivating prospect revealed in this report is the emergence of Generative AI as the next frontier in PV. The data echoes the industry's collective anticipation of its potential, signaling a transformational shift in how drug safety is approached. However, this anticipation comes with the need for addressing concerns about data privacy, regulatory compliance, and validation processes - a balancing act that promises immense rewards for those who dare to explore.
The report culminates in a compelling set of recommendations, urging life sciences companies to seize the opportunities unveiled. It advocates for strategic investments in technology, deliberate alignment of vendors with long-term visions, proactive exploration of generative AI, and an astute evaluation of outsourcing strategies. These recommendations serve as a blueprint for future-ready drug safety operations, driving efficiency and scalability and, ultimately, enhancing patient well-being.
In a world where every advancement in drug safety directly impacts patient lives, this report stands as a testament to the industry's commitment to continuous improvement. It's a rallying call to embrace change, harness the power of technology, and chart a course toward a future where drug safety isn't just a practice but a relentless pursuit of excellence and better health outcomes for all.
Dive into this report and uncover trends that are propelling drug safety into a new era of possibility and promise.