Digital technologies designed to support clinical trial management have evolved greatly over the years, spurred by increasing complexity in study design, emerging technologies in specific therapeutic areas, and a list of subtly different global regulations. Yet many eClinical technologies have remained unoptimized for user experience, even as technologies supporting other industries have continued to respond to the innovations led by more far-reaching, consumer-driven applications, websites, and platforms that typify what users have come to expect from their digital experience.
Although the overarching goal of collecting necessary data for a trial’s success has remained unchanged since the first eClinical technologies were deployed, the subtleties surrounding participant expectations, data integrity, and data collection have shifted significantly. The longstanding drive to achieve “equivalence” – to map electronic Clinical Outcome Assessments as closely as possible to their paper counterparts – has served to restrict and impede the design of these platforms. The result has been a digital landscape of flat, sterile interfaces that lack the familiarity and ease of use that could enhance their usability and engagement. Achieving better, more accessible eClinical technologies requires exploring the needs, motivations, and preferences of users in order to understand how participants interact with these technologies, as well as how developers can design solutions to compete with best, most cutting-edge apps and online interfaces on the market today.
Research-Driven Design for Better User Experience
More than 20 years ago, when the first electronic diaries began to emerge in clinical trial applications, their utility was meant to improve upon that of an analog diary, which afforded trial teams no means of determining when entries were completed. With paper diaries, a participant could choose to complete multiple entries at once, days or weeks after the date in question, potentially compromising the completeness or accuracy of the information conveyed. To circumvent this issue, the first generation of electronic diaries were designed to record time series data, electronically stamping diaries as information was entered. Approaches like these, aimed at streamlining and improving data collection and accuracy, represented some of the first eClinical technologies in the market; at the time the technology used was novel to trial teams and users alike.
Today, the digital landscape has changed – painstakingly curated content, more intuitive interfaces, and streamlined visual design have converged to create a user experience well beyond what was possible 10 or 20 years previous. As a result, those participating in clinical trials may notice a distinct gap between the user experience they’re accustomed to and the one they have when interacting with eClinical technologies, many of which remain unoptimized for usability. In order to better understand the wants and needs of participants who are more technology savvy than ever, providers, sponsors and trial teams should pursue insights into how people interact with digital technologies, as well as whether eClinical design solutions meet their needs and expectations.
Research-driven design enables this level of understanding through interviews, surveys, usability testing, and other feedback methodologies geared toward identifying solutions that offer users a better, more navigable, more engaging interface. This means moving beyond time stamps, built-in logic, or other baseline improvements to paper – research-driven design can help establish a more intuitive, familiar interface for clinical trial participants that better captures and keeps their attention. This is crucial for the clinical trial space, as eClinical technologies can go a long way toward making – or breaking – recruitment and retention. In a paradigm where recruiting and retaining participants are among the primary reasons trials stall or terminate, testing and validating eClinical approaches through research-driven design can offer sponsors important tools for ensuring they achieve their trial’s ultimate goals.
User Survey: Product and Experience Insights to Inform UX Design
YPrime, a technology provider focused on solutions that simplify clinical trial management, has long prioritized platform optimization for its partners through continuous improvements to its eClinical platforms. Part of this iterative improvement has been informed by research-driven design, with large-scale surveys, user testing, and smaller, more detailed interviews with clinical trial participants. To establish a baseline understanding of participant needs as they relate to eClinical technologies, YPrime first launched an online survey that netted more than 1,600 clinical trial participants from across multiple countries and disease indications. This survey, comprising 26 questions, sought insights related to participant demographics and health, technology experience and usage, clinical trial participation preferences, communication preferences, and attitudes surrounding digital engagement. Participants in the survey hailed primarily from Germany, the U.K., the U.S., and Spain, were predominantly female, and averaged 56 years of age.
Most respondents indicated that they spent an average of 3 to 5 hours a day on their phones; additionally, most indicated that they sometimes or always changed the settings within an application or platform to customize their user experience. Additional questions related to what aspects of a user interface respondents were most likely to customize; respondents overwhelmingly indicated features such as notification sounds, screen brightness, and font size as features they frequently customized. Likewise, when asked what features were important to them, respondents answered that ease of use around navigation and sign in were very important, as was the ability to manage alert notifications. Overall, app speed and performance were signaled as particularly important to users and the need to repeatedly input passwords was flagged as burdensome.
User Testing and Research: eCOA and eConsent
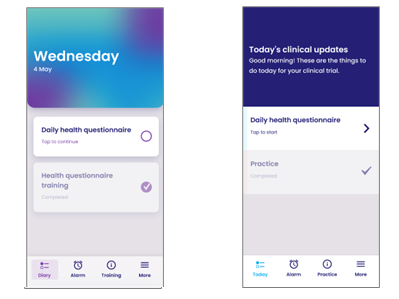
Of YPrime’s initial eCOA app prototypes, Version A (left) scored an 8.94 and Version B a 7.89 on a scale of 1 to 10 among polled users.
Following its initial survey, YPrime launched user testing aimed at evaluating and comparing prototypes of the latest version of its eCOA platform. Users were asked to evaluate two versions of the eCOA interface, navigating through everything from logging in to completing questionnaires to submitting data, offering feedback on the overall look and feel of each. While users indicated that both versions were easy to navigate, clear, straightforward, and easy to read, the majority specified a preference for Version A, describing it as “brighter and more vibrant.” Others indicated that they preferred the date be visible on the home page, as in Version A, and noted that the color scheme of Version B felt colder and “reminded them too much of a hospital.” Overall, user testing indicated that the font size and color contrast of Version B were poor and inconsistent when compared to Version A.
User research, in the form of one-on-one interviews, was also conducted based on initial eConsent drafts. YPrime conducted 40 in-depth interviews in subjects’ native languages, focusing on many of the same variables evaluated during user testing. In these interviews, participants indicated that the eConsent draft was overall easy to read and understand, and its structure and layout were viewed favorably. While interviewees highlighted which icons were universally recognizable, others pointed to some icons, such as the one linked to the document’s table of contents, as less familiar, prompting YPrime to make changes in the final design.
Conclusion
Ultimately, YPrime utilizes findings from its surveys, interviews, and user testing to inform iterative design improvements for its platforms. Using insights gained from research-driven design, YPrime is able to combine the best features of each design as identified by participants, repeating testing as needed to generate better, more accessible user interfaces. By focusing on both how participants interact with other technologies more broadly and what features and functionality they like and dislike in an eClinical technology, YPrime can inform both the next version of an application and the next phase of its research to improve those technologies more comprehensively. This proactive design approach is critical in an industry beset with complexity and populated with patients whose conditions and disease states can make their participation in a clinical trial challenging even under the best circumstances. In prioritizing patient experience when updating and designing eClinical technologies, YPrime works to design platforms with the necessary flexibility and user experience to drive engagement and provide technology better suited to the needs and expectations of our end users.