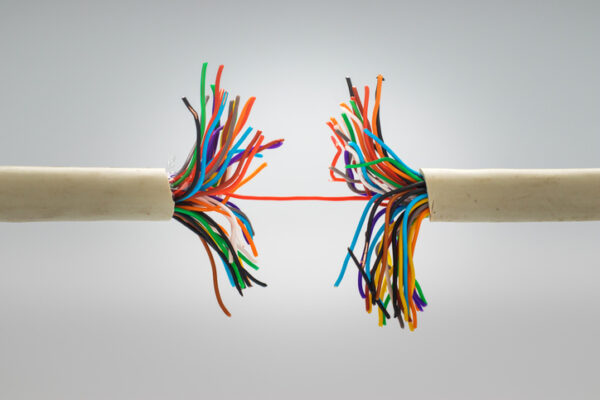
Safety is paramount in medical care, rooted in the most basic tenet of medicine: First, do no harm. But, although acute medical care presents myriad risks to patient safety, there is no category for devices designed to preserve that safety.
For one example, take intravenous (IV) access, which is required annually by an estimated 90 percent of acute care patients in the U.S.; some 342 million Americans require peripheral IV catheters alone each year. Of those, 12 percent and 69 percent will dislodge and fail, interrupting therapy and posing significant safety risks to patients – and hitting the healthcare system for more than $2 billion. According to a study published in JAVA, 95 percent of 1,561 U.S. clinicians surveyed agreed that IV dislodgements continue to pose significant safety risks for patients and hospitals. Estimated events per year are more than five million.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
Published studies prove that securement alone, in any form, does not prevent accidental dislodgement of intravenous catheters for peripheral or central devices in any patient population. These dislodgement events contribute to poor patient outcomes, such as phlebitis and infection. Although the prevalence of central venous catheter dislodgement is lower than with peripheral catheters, the associated complications have been shown to be potentially more severe, because of the risk of serious bleeding, contamination leading to infection, and risk of air emboli. One study found that “[c]entral venous device-associated complications are a preventable source of patient harm, frequently resulting in morbidity and delays to vital treatment,” noting that “[d]ressings and securement products are used to prevent infectious and mechanical complications, however current complication rates suggest customary practices are inadequate.”
Apart from the potentially drastic adverse effects on patient outcomes, dislodgement also has cost implications; as noted above, peripheral IV dislodgement alone is estimated to cost $2 billion annually in the U.S. In most situations, dislodgement requires replacement with a new catheter, instantly doubling costs. However, research shows that second insertion attempts, for peripheral catheters especially, may take more than one attempt, driving costs up even further and causing increased trauma and risk to the patient. With central venous catheters, concerns about increased costs are compounded by insertion risk to the patient, including loss of vessels that may be required for downstream treatment.
From a risk perspective, providers can be litigation targets based on adverse events resulting from catheter dislodgement. This is especially true because the standards and guidelines addressing prevention and response are well-established, and technologies exist to address and mitigate the problem. Medical malpractice standards change because of changes in technology, not because of changes in the law, and clinicians who fail to keep current with both guidelines and new technologies – even before that technology is accepted as the standard of care – are exposed to liability risk.
Moreover, medical professionals have a legal responsibility and a moral duty to safeguard patients, instituting processes and technologies where common complications and problems have been identified to help to prevent them from happening again. If safety devices and techniques for intravenous access catheters can help reduce complications such as dislodgement, there is a responsibility to incorporate them into routine use to protect patients.
Fortunately, there are simple, cost-effective, and easy-to-use technologies that address this problem, including one that’s been shown in a clinical simulation study to prevent IV dislodgement up to 92 percent of the time. When placed between the existing IV catheter extension set and general IV tubing, these safety release valves protect the patient by separating as the pull tension on the IV tubing and catheter exceeds a predetermined threshold. When activated, the valves create a sterile seal for both sides of the fluid pathway, preserving the IV catheter for continued use.
Creating a category for technologies like the safety release valve would confer benefits, including improved patient safety, reduced costs for the healthcare system overall, and lowered liability risk. It would also encourage the development of more safety devices. Most importantly, establishing a safety device category would help fulfill modern medicine’s moral duty to safeguard the safety of the people it treats.
Nancy L. Moureau, RN, PhD, BSN, CRNI, CPUI, VA-BC, is CEO and Educational Specialist for PICC Excellence, Inc., which provides education, consulting, and research on vascular access devices. Dr. Moureau is an internationally recognized speaker on Peripherally Inserted Central Catheters (PICCs) and issues of vascular access practice.
Russ Nassof, JD the Founder and EVP of RiskNomics LLC, has more than 25 years of experience in environmental/healthcare risk consulting, focused on infection control, microbial contamination, toxic tort risk management, insurance defense, wildfire impact claims, and industrial hygiene.














