Continued process verification (CPV) is the third stage of the product lifecycle of any medicine either within the small or large molecule arena. The CPV is the subsequent step to process design (Stage 1) and process qualification (Stage 2).
The focus of the third stage is to monitor the manufacturing data (process parameters, material attributes, IPCs and others) and product quality to determine process variability, ensuring the manufacturing process is running as expected and within the defined control limits. A good CPV plan has been considered a vital tool for pharma and biopharmaceutical process control and to ensure manufacturability consistency, as well as to signal opportunities for process improvement.
Digital Continued Process Verification
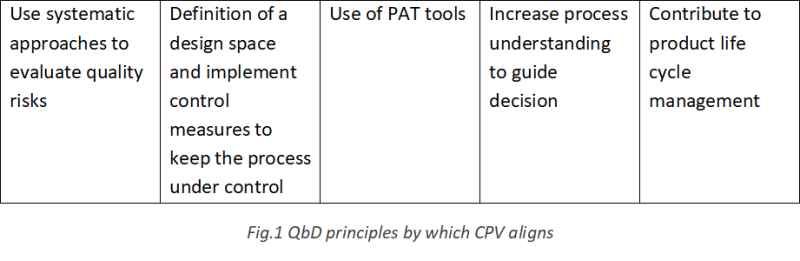
A well-maintained CPV program facilitates the understanding and identification of the variability of the manufacturing process by continuous collection of process data, thus, contributing towards increasing process knowledge and assuring manufacturing robustness, in line with the Quality by Design (QbD) framework (Figure 1). A CPV plan is a key component of a well-maintained process control strategy, contributing to the risk mitigation strategy of the process, yielding a more robust process and providing confidence on the manufacturing process to the regulatory authorities.
Nevertheless, maintaining a manual CPV plan is challenging, mainly due to the workload necessary to collect and organize the data, build efficient metrics and plots and update them with new data. The process of generating reports is also labor intensive and most of the tasks of data extraction, analysis, and collation are repetitive. However, they fall under the responsibility of highly skilled manpower with impact on cost.
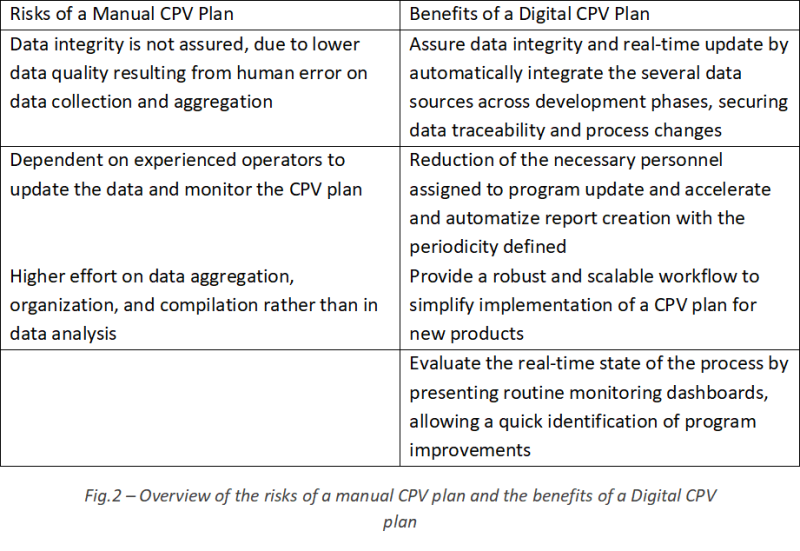
Overall, a manual CPV plan provides a reactive approach to problems that may arise, and opportunities for improvement might take longer to be identified. A digital CPV plan allows to focus on process improvements and proactive behavior.
Continuous Manufacturing
The FDA considers continuous manufacturing to be “a process in which the input material(s) are continuously fed into and transformed within the process, and the processed output materials are continuously removed from the system”.
When compared with traditional batch and fed-batch processes, continuous manufacturing has the following advantages:
- More flexibility on addressing supply demands.
- Increase in volumetric yields due to process intensification, which leads to less batches being needed and consequently also less waste generated.
- Continuous harvesting of the product, which is an advantage for molecules that may lose their quality attributes if the residence time is too long.
- Overall cost savings (after the initial investment) due to lower production and personnel costs and a smaller plant footprint resulting from optimized facility usage and reduced equipment size. On average, cost savings of up to 55% are expected when compared to traditional batch processes.
Still, these advantages come with a cost: continuous processes have a more complex operation and need an in-depth process understanding to achieve and maintain the steady-state conditions.
Modeling strategies can be used to increase process understanding, comprehend the complex correlations between process parameters and the product outcome and develop adequate control models.
These models might be data driven, mechanistic or hybrid approaches combining the latter two. Due to the myriad of parameters to control in such intricate processes, the use of AI algorithms such as artificial neural networks, support vector machines and random forests can be used to increase the control accuracy.
Digital CPV for Continuous Manufacturing
Currently, the application of CPV plans to continuous production processes is challenging due to the rising amount of data generated and the complex nature of a continuous process.
The steady stage of a continuous manufacturing process requires a robust control strategy where the speed at which measurements are acquired is crucial to address proactively process performance and quality deviations. Therefore, PAT systems play a key role when designing the control strategy. With biopharma processes, the complexity of designing a CPV Plan increases due to the variability inherent to living microorganisms, the challenges in modeling microorganism behavior with raw material quality and process parameterization and the complexity of the analytical methodology employed.
The continuous nature of the process means that there is no possibility to segregate a batch or a set of batches and assess impact on its quality. This lack of boundaries amplifies the importance of taking a proactive rather than reactive behavior, preventing the process from reaching “points of no return”, making the quality impact assessment complex and potentially impacting product release timelines to supply the market as well as leading to increased rejection rates of material.
Implementation of digital CPV plans can contribute to the control of continuous processes, by continuously analyzing process data in a multivariate way, allowing early detection of process faults and define in a timely fashion adequate corrective measure.
As previously mentioned in this article, the usage of process models to control the process can also be helpful to detect anomalies, such as general system perturbations, equipment malfunctioning and differences regarding process performance when compared to historical product quality and performance, in batch (for example by comparison with “golden batches”) or continuous mode.
Since continuous processes usually operate for extended periods of time, the probability of process and equipment anomalies is increased when compared to batch processes.
Quality issues such as process or product contaminations are also harder to assess its impact in the material being