Medical device and MedTech insights, news, tips and more
FDA greenlights Boston Scientific’s Novel Drug-Coated Balloon for Coronary In-Stent Restenosis
March 1, 2024
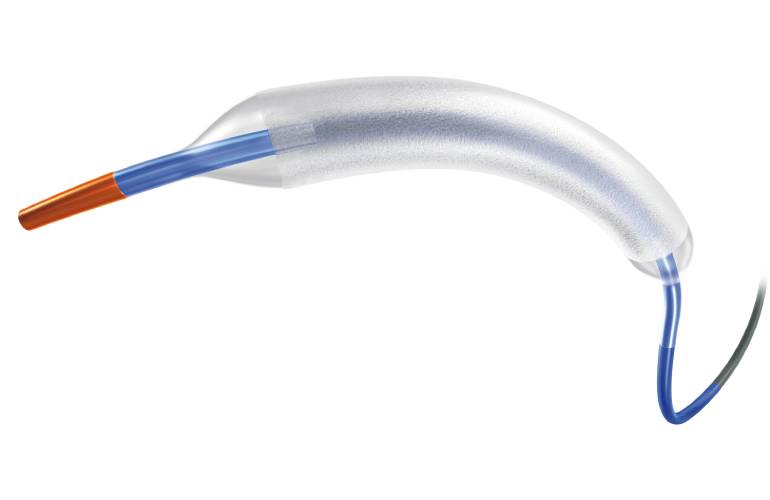
Boston Scientific’s AGENT™ Drug-Coated Balloon (DCB) has been granted approval by the U.S. Food and Drug Administration (FDA) for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease.
While advancements in percutaneous coronary intervention (PCI) technologies are ongoing, recent research indicates that ISR, the gradual re-narrowing of a previously opened coronary artery, still occurs in up to one in 10 patients post-treatment. The AGENT™ DCB aims to address this issue by specifically targeting the scar tissue with a therapeutic dose of the anti-proliferative drug paclitaxel, thereby preventing ISR from recurring. This device provides interventional cardiologists with an alternative treatment option to repeat PCI or cardiac surgery.
Lance Bates, Boston Scientific’s president of interventional cardiology therapies, expressed satisfaction at the introduction of this proven therapy as the first drug-coated coronary balloon in the U.S. He stated, “The AGENT™ DCB addresses a critical unmet need by providing a dedicated treatment option for the challenging condition of ISR, and we look forward to offering U.S. physicians the opportunity to treat their patients with this novel device.”
The FDA’s approval was largely based on data from the AGENT™ IDE trial, which compared treatment with the AGENT™ DCB to traditional balloon angioplasty. Initial findings from AGENT™ IDE, presented by interventional cardiologist Robert W. Yeh, MD, MSc, MBA, at TCT 2023, showed that target lesion failure after one year was observed in 28.7% of balloon angioplasty patients and 17.9% of AGENT™ DCB patients. The Boston Scientific device was also associated with a reduced risk of stent thrombosis, target lesion revascularization, and target vessel myocardial infarction.
American College of Cardiology President B. Hadley Wilson, MD, described the results as a “game changer.” He noted, “For 25 years, we’ve been trying to peel back this restenosis problem. Now we can see light at the end of the tunnel. This is really terrific work.”
The final results from AGENT™ IDE are expected to be presented at CRT 2024.
The AGENT™ DCB has been available in Europe and other parts of the world for several years. Boston Scientific plans to launch this newly approved interventional device in the United States “in the coming months.”
See Full Press Release at the Source: Boston Scientific Receives FDA Approval for the AGENT™ Drug-Coated Balloon
Press Release by: Boston Scientific Corporation

Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 19 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
