Industry Predictions For 2023
PharmExec
DECEMBER 28, 2022
Top industry experts weigh in on what the new year holds for the pharma industry.

PharmExec
DECEMBER 28, 2022
Top industry experts weigh in on what the new year holds for the pharma industry.

Fierce Pharma
DECEMBER 29, 2022
Biogen, FDA's inappropriate Aduhelm coordination detailed in blistering congressional report. aarmstrong. Thu, 12/29/2022 - 14:42.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
DECEMBER 26, 2022
By easing regulatory requirements for animal testing, the Act allows scientists to use innovative, leading-edge technologies more fully in future drug development strategies. .

Pharmaceutical Technology
DECEMBER 27, 2022
The healthcare industry saw its share of ups and downs in 2022. Our response to the worldwide pandemic evolved over time, and so did the needs of the sector and the people relying on healthcare companies to deliver solutions. Along the way, mergers and acquisitions continued to happen, new drugs and devices got approved, and innovations in the clinical trial industry were introduced.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

pharmaphorum
DECEMBER 28, 2022
This year, the predicted flurry of merger and acquisition (M&A) activity might not have materialised. However, as we near the end of the year, there were some big moves. M&A is an integral part of the lifecycle of pharma companies and a key strategy to future-proof larger players, driving R&D activities and innovation for a competitive product pipeline.

Fierce Pharma
DECEMBER 27, 2022
Roche gets FDA nod for Lunsumio, offering convenient option to gene therapies in follicular lymphoma. kdunleavy. Tue, 12/27/2022 - 09:16.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
Medgadget
DECEMBER 27, 2022
Researchers at Binghamton University have developed a microbial fuel cell that can power ingestible devices, such as cameras, that can detect health issues in the gastrointestinal tract, and specifically within the small intestine. The fuel cell contains dormant Bacillus subtilis endospores that only germinate and become active when they encounter nutrient-rich intestinal fluid.
pharmaphorum
DECEMBER 29, 2022
The turbulence of the last three years can make it seem as though pharma manufacturers have been operating in an atmosphere of non-stop crisis. COVID-19 erupted, mutated, and was supplemented by the challenges of monkeypox and the need to catch up with major treatment and immunisation backlogs accumulated during the pandemic. At the same time, the industry must address the long-term difficulty of providing essential medicines and health services to the high percentage of global population that c
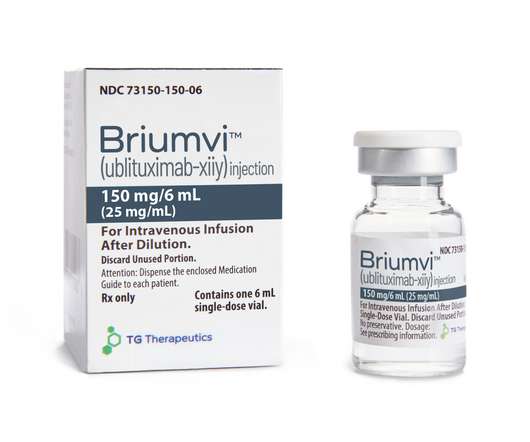
Fierce Pharma
DECEMBER 29, 2022
After FDA's Ukoniq pull, TG Therapeutics wins commercial mulligan with Briumvi to enter crowded MS arena. fkansteiner. Thu, 12/29/2022 - 09:30.

MedCity News
DECEMBER 28, 2022
The industry needs to more clearly define the different competencies and career paths within clinical research so all employees can lean into their strengths and build a career plan that brings them fulfillment.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharma Pathway
DECEMBER 28, 2022
Macleods Pharmaceuticals Ltd.-Openings for Engineering Services-Apply Now. Job Description. Macleods Pharmaceuticals, Indian’s fastest growing company was established in 1986. Ranked amongst the top 10 pharma companies with a strength of 20000 employees, operating in 140+ countries. Our bioequivalence centre was established in 2005 and is responsible for conducting studies for filing product registrations with various regulatory authorities.

pharmaphorum
DECEMBER 30, 2022
The vast majority of GPs are persuaded of the benefits of moving diagnostic services from the hospital to the community, according to a new survey of 200 GPs in the UK. Just 4% of would choose the hospital as the preferred location of testing, compared to 46% for the GP surgery, 18% for a community hub, 15% for a patient’s home and 12% for the pharmacy.

Fierce Pharma
DECEMBER 28, 2022
UCB's bimekizumab finally back on the FDA's review list after May rejection. zbecker. Wed, 12/28/2022 - 11:17.

MedCity News
DECEMBER 27, 2022
Hospitals go to great lengths to deliver exceptional care to their patients. That commitment should extend to the digital space. By making your inventory visible, breaking down data silos, and building a better patient experience, providers will have happier patients and healthier margins.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr
Pharmaceutical Technology
DECEMBER 27, 2022
Indian pharmaceutical company Hetero has received the World Health Organization Prequalification of Medicines Program (WHO PQ) approval for its Nirmacom (nirmatrelvir). The company stated that Nirmacom is a generic version of Pfizer ’s Covid-19 oral antiviral drug, Paxlovid, a SARS-CoV-2 main protease (Mpro) inhibitor. Hetero’s Nirmacom combi-pack will contain nirmatrelvir 150 mg (two tablets) and ritonavir 100mg (one tablet).

pharmaphorum
DECEMBER 29, 2022
A new Data Protection and Digital Information Bill is currently being considered by UK ministers. The Bill has been created to update and simplify the UK’s data protection framework with a view to reducing burdens on organisations, providing them with greater flexibility on how to comply with certain aspects of the data protection legislation and improving the clarity of the framework.

Pharma Pathway
DECEMBER 28, 2022
Aurobindo Pharma-Openings for M.Pharmacy Freshers -Apply Now. Job Description. Opportunity for M.Pharmacy Freshers. Aurobindo Research Centre 2 at Pashamaylaram, Hyderabad Location is providing an Opportunity for M.Pharmacy Freshers to gain the Industrial knowledge through its 9 months internship in the below R&D Areas. Formulations R&D (OSD): M.

MedCity News
DECEMBER 28, 2022
Carallel, a startup that provides digital tools to support family caregivers, recently raised $8.2 million in Series A funding. The Chicago-based company’s services are offered as a benefit by health plans and employers.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Copyright Clearance Center
DECEMBER 26, 2022
The post Top Stories of 2022 for Books and Reading appeared first on Copyright Clearance Center.

pharmaphorum
DECEMBER 30, 2022
Speaking with Rob Verheul, CEO of Graphite Digital, at Anthropy 2022 at the Eden Project, pharmaphorum web editor Nicole Raleigh enjoyed a minute discussing the event and what specifically had attracted Verheul to attend. Chatting about Graphite Digital’s role as a design specialist for HCPs, patient groups, and ‘everything in between’ – the vision and purpose that encircles that – Verheul and I look at the conversations being sparked at the event, the challenges that are clear across industries

Pharma Pathway
DECEMBER 29, 2022
Pfizer -Openings for MSAT (Manufacturing Science & Technology)- Apply Now. Job Description. Openings for MSAT (Manufacturing Science & Technology) @ Pfizer. Keen on building your career with Pfizer!!! . Looking Female Manpower to our Pfizer Global Supply (PGS) Vizag Plant. Department: MSAT (Manufacturing Science & Technology). Functional Areas: Analytical/ Process (Technology Transfer).

MedCity News
DECEMBER 28, 2022
Putting data into the hands of participants is an important step in changing the traditional clinical trial paradigm. It fits in amongst the goal of running nimble and patient-centered studies. But, without legislation, it’s doubtful that pharma companies will give up ownership of participant data out of sheer goodwill.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Commerce
DECEMBER 28, 2022
Observations from recent digital health events and a glimpse of what’s ahead—from Milan to Boca Raton.

pharmaphorum
DECEMBER 28, 2022
Biogen’s efforts to replenish its multiple sclerosis pipeline have suffered a setback, after the FDA placed a clinical hold on a drug candidate it licensed from China’s InnoCare last year for $125 million upfront. The US regulator has placed oral BTK inhibitor orelabrutinib on partial clinical hold, meaning new patients cannot be enrolled into clinical trials and patients treated with the drug for 70 days or less must discontinue treatment.

Pharma Pathway
DECEMBER 30, 2022
Zenfold Sustainable Technology (ZST)- Openings for Production / Stores Dept. -Apply Now. Job Description. Openings with Zenfold Sustainable Technology (Specialty Chemicals, API) for Production / Stores Departments. Department: Production / Stores. Position: Chemist/ Officer/ Executive. Qualification: B.Sc/ M.Sc/ B.Tech. Experience: 02 to 05 years.

MedCity News
DECEMBER 29, 2022
Our research has found that to advance ongoing work, community health ecosystems likely will need additional partners, shared goals, technology, and metrics. But most importantly, they will need the participation and leadership of more community members.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Pharmaceutical Technology
DECEMBER 29, 2022
The Japanese Ministry of Health, Labor, and Welfare (MHLW) has approved AstraZeneca ’s immunotherapies Imfinzi (durvalumab) and Imjudo (tremelimumab) to treat advanced liver, biliary tract, and lung cancers. The combination of Imfinzi and Imjudo has been approved to treat unresectable hepatocellular carcinoma (HCC) in adult patients. It has also been authorised to treat unresectable, advanced or recurrent non-small cell lung cancer (NSCLC) along with chemotherapy.
pharmaphorum
DECEMBER 30, 2022
The FDA’s interactions with Biogen in the build-up to last year’s controversial approval of Alzheimer’s disease therapy Aduhelm have been described as “inappropriate” and “atypical” in a congressional report on the review. The document produced by Oversight and Reform Committee chair Carolyn Maloney (D-NY) and Energy and Commerce Committee chair Frank Pallone Jr (D-NJ) also points to “collaboration” between Biogen and the FDA ahead of an advi

Pharma Pathway
DECEMBER 30, 2022
Alembic Pharmaceuticals Ltd- Openings for Trainee- IT Helpdesk – Apply Now. Job Description. Alembic Pharmaceuticals Ltd. is an Indian multinational Pharmaceutical company headquartered in Vadodara city of Gujarat-India. Alembic pharmaceuticals Ltd. is involved in manufacture of pharmaceutical products, pharmaceutical substances and Intermediates.

MedCity News
DECEMBER 26, 2022
If women are the influential guide for many to access healthcare, we must work harder to promote the health of all women. Our ability to play the role of care facilitator for others depends upon it.

Advertiser: ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.
Let's personalize your content