Technology as a New Evolution in Fighting Disease
PharmaTech
JANUARY 3, 2023
Advancing the use of AI to understand the whole of a disease can reveal drug development insights that lead to drug discovery breakthroughs.

PharmaTech
JANUARY 3, 2023
Advancing the use of AI to understand the whole of a disease can reveal drug development insights that lead to drug discovery breakthroughs.

Fierce Pharma
JANUARY 9, 2023
JPM23, Day 1: BMS touts new launches as patent cliff looms; Regeneron's Eylea sales disappoint. esagonowsky. Mon, 01/09/2023 - 09:55.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
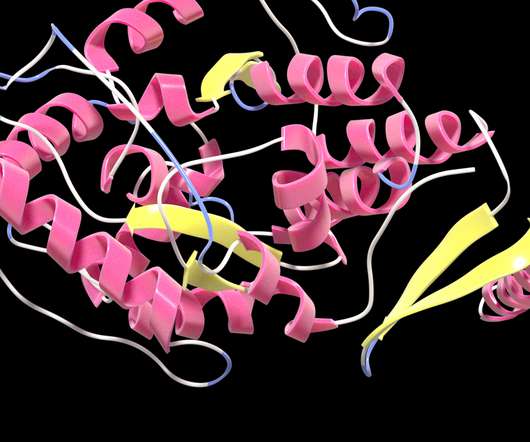
MedCity News
JANUARY 22, 2023
A collaboration between Nvidia and startup Evozyne was able to produce novel versions of a human protein never before seen in nature but with enhanced function and the same safety as native protein. The research lays the groundwork for potential new therapies for a rare inherited disorder.

pharmaphorum
JANUARY 6, 2023
Google and DeepMind have developed an artificial intelligence-powered chatbot tool called Med-PaLM designed to generate “safe and helpful answers” to questions posed by healthcare professionals and patients. The tool is an example of a large language model or LLM, which are designed to understand queries and generate text responses in plain language, drawing from large and complex datasets – in this case, medical research.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
JANUARY 13, 2023
Health Canada has granted approval to Enhertu (trastuzumab deruxtecan) to treat unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer. Enhertu has been approved to treat HER2-low breast cancer adult patients who have previously received at least one line of chemotherapy in the metastatic setting or who have seen disease recurrence during or within six months after the adjuvant chemotherapy.
European Pharmaceutical Review
JANUARY 23, 2023
The US Food and Drug Administration (FDA) has granted fast track designation (FTD) for Evaxion Biotech’s personalised cancer immunotherapy. The FTD is for EVX-01, in combination with Keytruda ® for patients with metastatic melanoma (MM). ”We are extremely pleased that our cancer vaccine candidate EVX-01 has received the FDA fast track designation, as it enables a potentially faster approval of the vaccine,” stated Per Norlén, CEO at Denmark-based Evaxion.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
Fierce Pharma
JANUARY 17, 2023
CDC probes possible safety risk for Pfizer's new COVID shot, sees no need to change vaccine practices. zbecker. Tue, 01/17/2023 - 11:49.

MedCity News
JANUARY 12, 2023
UC Davis Health announced the first startup to come out of its health tech innovation incubator. The company, named WellCent, is a platform that allows patients and caregivers to onboard medical devices into their home and access digital health resources.

pharmaphorum
JANUARY 27, 2023
AstraZeneca’s revenue boost from COVID-19 therapy Evusheld looks set to be curbed early, as the FDA withdraws authorisation for the antibody on the grounds that it is ineffective against most subvariants now circulating in the US. Evusheld (tixagevimab and cilgavimab) was cleared by the FDA towards the end of 2021, becoming the first antibody to be authorised for prevention of COVID-19 infection, and it rapidly found use among people with compromised immune systems, such as cancer chemothe
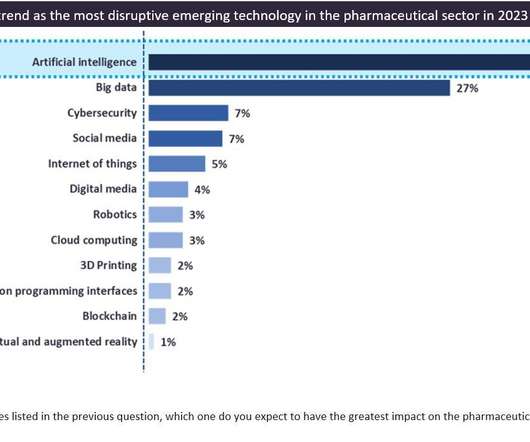
Pharmaceutical Technology
JANUARY 16, 2023
While the biopharmaceutical industry has been impacted and pressured by various factors such as the Covid-19 pandemic, inflation, the Ukraine-Russia war, ongoing supply chain issues, and a challenging economic environment, collaboration between pharma companies and emerging technologies providers continues to grow, especially in the research and development (R&D) field, offering some resilience in times of geopolitical and economic disruptions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

European Pharmaceutical Review
JANUARY 26, 2023
Yescarta ® ▼(axicabtagene ciloleucel; axi-cel) is now the first chimeric antigen receptor (CAR) T-cell therapy and first personalised immunotherapy to be recommended for routine use on the NHS in England. This is based on final draft guidance from the National Institute for Health and Care Excellence (NICE). The therapy by Gilead Sciences and Kite is indicated for eligible adults with diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) who have already bee

PharmaVoice
JANUARY 3, 2023
Durect’s chief medical officer altered his retirement plans to take a leading role at the company, which is developing a treatment for severe alcohol-associated hepatitis.

Fierce Pharma
JANUARY 26, 2023
BMS settles lawsuit with two fired employees who refused COVID vaccines kdunleavy Thu, 01/26/2023 - 14:30
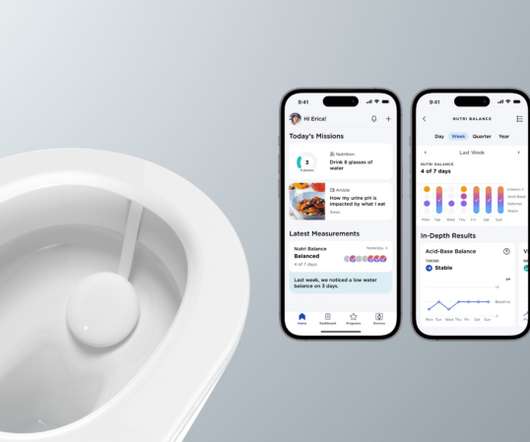
MedCity News
JANUARY 3, 2023
Withings announced that it is developing a miniaturized platform that can analyze urine at home. The device, called U-Scan, sits within a toilet bowl and assesses specific biomarkers found in urine.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

pharmaphorum
JANUARY 12, 2023
Bayer has signed an agreement with Google aimed at using high-level processing power to handle quantum chemistry calculations used to predict the chemical and physical properties of drug molecules at the atomic scale. The deal with the tech giant’s Google Cloud unit revolves around its tensor processing units (TPUs), artificial intelligence-powered accelerators designed to run machine learning models and computationally-intensive workloads that can be customised to specific applications.

Pharmaceutical Technology
JANUARY 8, 2023
In 2023, the pharmaceutical industry will mark 20 years since Xolair, an anti-IgE antibody, became the first biologic approved to treat asthma. Since then, the US FDA, EMA, and other agencies have approved several biologic antibodies targeting the inflammatory cytokines IL-4, IL-13, IL-5, and others for asthma. Approaches like bronchodilator inhalers focus on treating both asthma and chronic obstructive pulmonary disease (COPD).

European Pharmaceutical Review
JANUARY 23, 2023
There are major concerns about microbial contamination in cannabis, US Food and Drug Administration (FDA) researchers observed in a study. Multiple cases have been reported of infections associated with cannabis use caused by fungi and bacteria in immunocompromised individuals using inhaled cannabis material. Investigational New Drug (IND) applications for cannabis as therapeutics tested in clinical trials must comply with FDA’s requirements and standards for drug products.

PharmExec
JANUARY 19, 2023
Fran Pollaro talks with Amanda Powers-Han, chief marketing officer at Greater Than One, a full-experience marketing agency dedicated to healthcare, about what’s trending in early 2023 for pharma media mixes.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Fierce Pharma
JANUARY 9, 2023
JPM23: Moderna reaped $18.4B in COVID vaccine sales last year, projects at least $5B in 2023. kdunleavy. Mon, 01/09/2023 - 10:34.

MedCity News
JANUARY 12, 2023
California’s Office of the Attorney General contends that drugmakers and pharmacy benefit managers work together to “aggressively raise the list price of insulin in lockstep with each other to artificial levels.” Insulin manufacturers deny the allegations, pointing to availability of lower-cost versions of their products.

pharmaphorum
JANUARY 26, 2023
2022 was a banner year for genomics. In March, the collaborative T2T consortium published the first complete telomere-to-telomere sequence of the human genome, filling in the last 8% of the 3 billion base pairs that make up our DNA. And in the UK specifically, genomics remained high on the national agenda, with several significant government programmes and investments announced – including the Newborn Genomes Programme in healthcare and the Precision Breeding Bill in the agricultural sector.
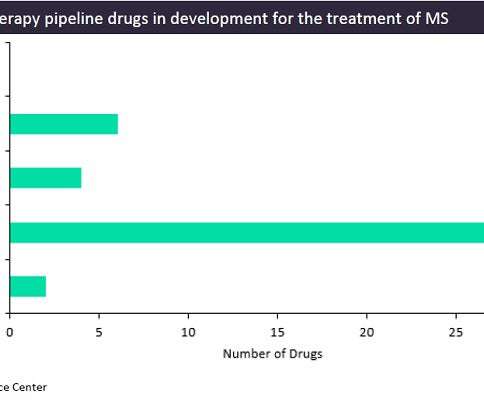
Pharmaceutical Technology
JANUARY 6, 2023
While the treatment options for multiple sclerosis (MS) patients are growing each year with the approval of new agents, all of the currently marketed treatments only slow the disease’s progression and sometimes carry risks of severe side effects, such as liver failure or the development of viral infections. However, new mechanisms of action (MoAs) are in constant development, with some of the more innovative ones utilizing cell-based therapies.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

European Pharmaceutical Review
JANUARY 11, 2023
Machine learning models used to guide the design of long-acting injectable drug formulations have been successfully tested by scientists at the University of Toronto. The research results signal the potential for machine to reduce reliance on trial-and-error testing, which slows the development of long-acting injectables (LAIs). The study was published Nature Communications and is one of the first to apply machine learning techniques to the design of polymeric long-acting injectable drug formula

PharmExec
JANUARY 11, 2023
As new forms of cell and gene therapies continue to be developed, life sciences companies and payers need to find alternative ways to pay for these expensive treatments. Value-based contracts, though slow to gain traction so far, may provide the solution these groups are looking for.

Fierce Pharma
JANUARY 25, 2023
Merck's Keytruda dealt another blow in prostate cancer but readies new challenge to AZ's Imfinzi aliu Wed, 01/25/2023 - 10:17

MedCity News
JANUARY 20, 2023
Healthcare could learn from the data improvement strategies that other industries have implemented in the past decade, a new report said. For example, the military, aerospace industry and aviation sector have all developed ways to standardize data, decrease silos and make information more accessible between organizations.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
pharmaphorum
JANUARY 11, 2023
Over the past few decades, data generation has veritably exploded. However, the ‘Big Data paradigm’ is not so much concerned with the volume of that data, but how businesses and, indeed, industries can derive meaningful insights from what has become a glut of information. With the currently popular approach to artificial intelligence (AI) focussing on the Big Data paradigm, also, pharmaphorum spoke with Adityo Prakash, CEO of Verseon, about the whys and wherefores, delving deeper into the proces
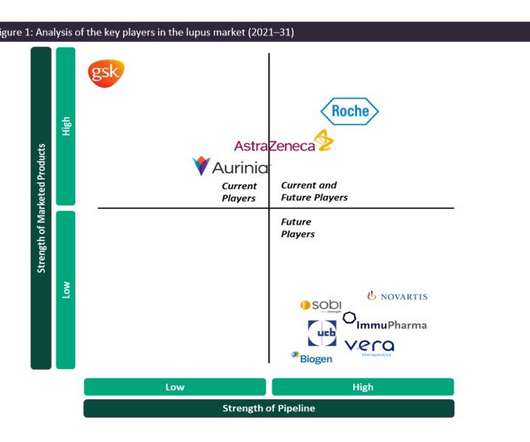
Pharmaceutical Technology
JANUARY 23, 2023
Systemic lupus erythematosus (SLE) is a systemic, inflammatory, chronic autoimmune disease that can affect multiple organs simultaneously or sequentially, with a relapsing and remitting nature. While SLE can affect multiple major organ systems in the body, one of its most severe manifestations is renal (kidney) involvement, known as lupus nephritis (LN).

European Pharmaceutical Review
JANUARY 16, 2023
A Mutual Recognition Agreement (MRA) relating to pharmaceutical Good Manufacturing Practice (GMP) has been signed between Switzerland and the US. Under the agreement with the Swiss Confederation (Switzerland), the Swiss Agency for Therapeutic Products (Swissmedic) and the US Food and Drug Administration (FDA) will be able to utilise each other’s GMP inspections of pharmaceutical manufacturing facilities, avoiding the need for duplicate inspections.

Healthcare Success
JANUARY 25, 2023
It’s not your imagination. People really are ruder to each other these days. The pandemic, economy, political unrest, war, and continued uncertainty have taken—and continue to take—a toll on people’s mental health. These all contribute to stress, anxiety, frustration, and anger. And according to Harvard Business Review , frontline workers are taking the brunt of this unrest and aggression.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content