From chemistry to canvas: What the pharma world can learn from art
PharmaVoice
DECEMBER 22, 2022
Art and science are often viewed as diametric opposites, but these industry insiders say their passion for painting blends into their pharma work.

PharmaVoice
DECEMBER 22, 2022
Art and science are often viewed as diametric opposites, but these industry insiders say their passion for painting blends into their pharma work.

Fierce Pharma
DECEMBER 22, 2022
Pfizer, Sanofi settle first California Zantac case slated for trial: report. zbecker. Thu, 12/22/2022 - 12:38.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaVoice
DECEMBER 1, 2022
Why South Rampart Pharma’s novel new molecule could be a key to solving the global pain epidemic.

Medgadget
DECEMBER 7, 2022
At the University of South Australia, researchers designed a system that allows them to measure a patient’s blood pressure with a camera. The camera visualizes the patient’s forehead and focuses on two regions in particular to optically determine photoplethysmographic signals that AI algorithms then convert to blood pressure data. The researchers tested their system in 25 volunteers, and so far it has proven to be approximately 90% as accurate as traditional pressure cuff measurements.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
pharmaphorum
DECEMBER 19, 2022
A collaboration between academic centres in the UK has won government funding for a digital approach to dysfunctional breathing or dyspnoea, a symptom that affects around 10% of the population. The Engineering and Physical Sciences Research Council (EPSRC) has set aside £400,000 (almost $490,000) for the project, which will help fund work at the Universities of Plymouth, Salford and Stirling, and the Glasgow School of Art.

Pharmaceutical Technology
DECEMBER 21, 2022
Moderna and the UK government have entered a ten-year strategic collaboration to build a messenger ribonucleic acid (mRNA) research, development and manufacturing facility in the country. The latest development comes after the parties announced an agreement in principle in June this year. This Moderna Innovation and Technology Centre (MITC) is expected to offer access to a locally produced future mRNA vaccine portfolio against respiratory viruses, subject to regulatory evaluation and licensure.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Fierce Pharma
DECEMBER 29, 2022
Biogen, FDA's inappropriate Aduhelm coordination detailed in blistering congressional report. aarmstrong. Thu, 12/29/2022 - 14:42.

MedCity News
DECEMBER 12, 2022
It may take some time to introduce VR into your skilled nursing facility, but it will be worth it for the physical, mental, and social benefit of your residents.

European Pharmaceutical Review
DECEMBER 22, 2022
Moderna, Inc. has finalised a strategic partnership with the UK government to establish a state-of-the-art mRNA vaccine research, development, and manufacturing facility in the UK. This milestone follows the agreement in principle between Moderna and the UK Government, announced in June 2022. The Moderna Innovation and Technology Centre (MITC) is intended to provide access to a UK-made supply of COVID-19 jabs.

pharmaphorum
DECEMBER 11, 2022
Digital transformation in pharma is not a singular endeavour. It can mean everything from patient-facing disease management apps and wearables to background AI dramatically altering drug discovery or radiological imaging. So, when a life sciences company embarks on a project of digital transformation or innovation, it’s really embarking on multiple projects that span the wide world of pharmaceutical operations.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
DECEMBER 23, 2022
Eli Lilly and Company has expanded a licencing and partnership agreement with ProQR Therapeutics to discover, develop and market new genetic medicines. The companies entered the initial agreement in September last year. This alliance is utilising the Axiomer ribonucleic acid (RNA) editing platform of ProQR to address ailments affecting the liver and nervous system.
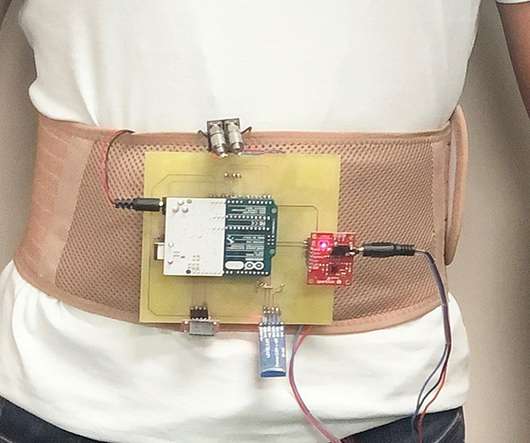
Medgadget
DECEMBER 21, 2022
Researchers at Florida Atlantic University have developed a belt that can monitor heart failure patients for signs of disease progression. The wearable device measures heart rate, thoracic impedance, electrocardiogram, and motion, all of which can provide information on a heart failure patient’s status and potentially enable early detection of disease exacerbation.
Fierce Pharma
DECEMBER 13, 2022
Cancer center leaders lay bare CAR-T makers' struggles—and an unexpected laggard. aliu. Tue, 12/13/2022 - 20:12.

MedCity News
DECEMBER 26, 2022
By easing regulatory requirements for animal testing, the Act allows scientists to use innovative, leading-edge technologies more fully in future drug development strategies. .

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

PharmExec
DECEMBER 8, 2022
Our annual report spotlighting notable investments in new drug development captures a mix of gradual gains and giant leaps—both equally as promising—in five expanding and diverse therapeutic areas: spinal muscular atrophy, hemophilia A, intranasal and inhaled vaccines, gene-targeted therapy, and RNA therapeutics.

pharmaphorum
DECEMBER 7, 2022
Elon Musk’s brain computer interface (BCI) company Neuralink is being investigated by law enforcement authorities in the US amid allegations of animal welfare violations in its testing facilities. Neuralink’s BCI is intended to treat conditions like blindness and spinal cord injury, as well as provide a way to interact with digital devices using the brain and, according to Musk, is on the brink of moving into the human testing stage.

Pharmaceutical Technology
DECEMBER 27, 2022
The healthcare industry saw its share of ups and downs in 2022. Our response to the worldwide pandemic evolved over time, and so did the needs of the sector and the people relying on healthcare companies to deliver solutions. Along the way, mergers and acquisitions continued to happen, new drugs and devices got approved, and innovations in the clinical trial industry were introduced.

Healthcare Success
DECEMBER 6, 2022
Healthcare consumers continue to move swiftly down the paths of least resistance toward quality care. This spells trouble for healthcare organizations that cannot change with the times. It’s time to pay attention to what competitors offer and find ways to remove unnecessary obstacles to care. In this blog, I share nine healthcare marketing trends you need to embrace in 2023 to remain competitive in today’s ever-changing healthcare marketplace. 9 Healthcare Marketing Trends 2023. 1.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.
Fierce Pharma
DECEMBER 19, 2022
Atara makes history with world-first nod for allogeneic T-cell therapy Ebvallo. fkansteiner. Mon, 12/19/2022 - 14:06.

MedCity News
DECEMBER 28, 2022
As we collectively address this rising tide of behavioral health needs, we will need more payment reform, more innovation, and continued migration to more prospective payment models and treatment approaches that bring all stakeholders together to care for patients in a holistic manner.

PharmExec
DECEMBER 14, 2022
Perspectives from a board member and an aspiring board member on how to increase the number of qualified women on boards.

pharmaphorum
DECEMBER 22, 2022
Construction will start early next year of a new manufacturing centre in the UK with the capacity to produce 250 million vaccine doses per year, the centrepiece of a 10-year alliance between the government and US biotech Moderna. The government said today it has finalised the partnership – agreed in principle earlier this year and estimated to be worth in the region of $1.2 billion – although it is not revealing the financial details, as these are “commercially sensitive.” The overar

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Technology
DECEMBER 8, 2022
Kite Pharma and Daiichi Sankyo have updated a partnership agreement signed in 2017 for the former’s CAR T-cell therapy, Yescarta (axicabtagene ciloleucel). Under the prior deal, Daiichi Sankyo acquired exclusive rights for the development, manufacturing and commercialisation of Yescarta in Japan. Subsequently, in the same year, Gilead Sciences acquired Kite.

European Pharmaceutical Review
DECEMBER 21, 2022
Fluoxetine was approved to treat depression 35 years ago. Since then, there have been few breakthrough innovations in treating neuropsychiatric diseases such as anxiety, depression, substance use disorders (SUDs), and post-traumatic stress disorder (PTSD). For many indications, progress has been incremental. Psychedelic research, in particular, is gaining momentum. .

Fierce Pharma
DECEMBER 21, 2022
After overcoming vial issue, Gilead wins FDA approval for long-acting HIV injectable Sunlenca. zbecker. Wed, 12/21/2022 - 17:50.

MedCity News
DECEMBER 20, 2022
As with most industries, healthcare should consider adopting a zero-trust approach. This security measure can help decrease an organization’s attack surface, create accurate response automation and prevent the compromise.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

Medgadget
DECEMBER 16, 2022
This past week, Medgadget was an official media partner with the Healthcareᐩ Expo Taiwan. This four-day annual event brings together healthcare companies from all over Taiwan and the rest of the world. It is also an opportunity for tech companies not typically associated with healthcare, such as ASUS, Intel, and BenQ, to showcase what they’re doing in medicine and healthcare.
pharmaphorum
DECEMBER 16, 2022
CSL’s gene therapy for haemophilia B has been recommended for approval by the EMA’s human medicine committee, setting up a decision by the European Commission early next year. The positive opinion for etranacogene dezaparvovec – which was approved as Hemgenix by the FDA last month – raises the prospect of the first one-time therapy in the EU for the bleeding disorder, which affects around 1 in 50,000 of the population, according to the European Haemophilia Network (EUHANET).

Pharmaceutical Technology
DECEMBER 18, 2022
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended granting variation to the marketing authorization (MA) for Moderna ’s Spikevax bivalent Original/Omicron BA.1 (mRNA-1273.214) booster for usage in children aged six to 11 years. The 0.25mL dose of the booster vaccine could potentially be used in the European Union (EU) following authorisation in these children a minimum of three months following a previous Covid-19 vaccination.
European Pharmaceutical Review
DECEMBER 14, 2022
Moderna, Inc. and Merck have announced that a Phase IIb trial ( NCT03897881 ) of a personalised mRNA cancer vaccine (mRNA-4157/V940), in combination with KEYTRUDA ® , Merck’s anti-PD-1 therapy, demonstrated the first randomised evidence that a personalised neoantigen approach may be beneficial in treating melanoma. Adjuvant treatment with mRNA-4157/V940 in combination with KEYTRUDA ® reduced the risk of recurrence or death by 44 percent (HR=0.56 [95 percent CI, 0.31-1.08]; one-sided p-valu

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content