UK regulators warn of ocular side effects after treatment with Sanofi's Dupixent
Fierce Pharma
NOVEMBER 30, 2022
UK regulators warn of ocular side effects after treatment with Sanofi's Dupixent. zbecker. Wed, 11/30/2022 - 10:55.

Fierce Pharma
NOVEMBER 30, 2022
UK regulators warn of ocular side effects after treatment with Sanofi's Dupixent. zbecker. Wed, 11/30/2022 - 10:55.

MedCity News
NOVEMBER 30, 2022
Patient privacy is evolving rapidly in the post-Dobbs era, according to healthcare and life science lawyers in a webinar hosted by the American Bar Association on Wednesday. .
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
NOVEMBER 30, 2022
GSK unveils Keytruda head-to-head data in lung cancer—and they look good at first glance. aliu. Wed, 11/30/2022 - 16:32.

Pharmaceutical Technology
NOVEMBER 30, 2022
In what’s being called a “medical bypass”, new anti-obesity drugs are almost matching efficacy rates in weight loss that have previously only been seen with weight loss surgeries. In October, the US FDA granted Eli Lilly’s Mounjaro (tirzepatide) a Fast Track designation for its use as a treatment for obesity. The drug was approved to treat type 2 diabetes mellitus in May.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
NOVEMBER 30, 2022
Argenx hands bluebird $102M for FDA speedy review voucher. fkansteiner. Wed, 11/30/2022 - 09:34.

Spotio
NOVEMBER 30, 2022
Your sales commission structure is a critical piece of your company’s success. It sets the bar for the level of talent that you’ll attract to your sales team. It seems simple, right? More money = better salespeople? But this isn’t always true. Higher earning potential through a commission-only comp plan won’t necessarily outweigh the risk a salesperson inherits by not having a guaranteed income source.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
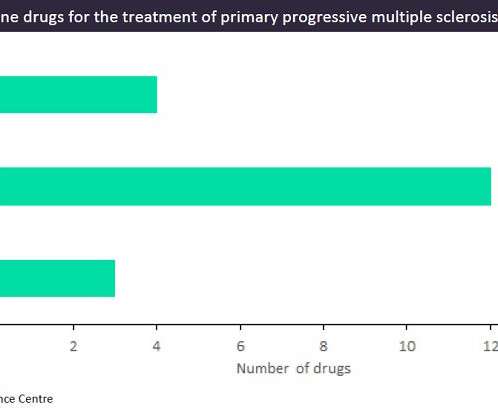
Pharmaceutical Technology
NOVEMBER 30, 2022
Multiple sclerosis (MS) is an autoimmune disease causing chronic inflammation and demyelination of the nerves, affecting around 2.5 million people worldwide. While patients diagnosed with relapsing MS (RMS) have a wide range of marketed treatment options, patients diagnosed with progressive MS, especially primary progressive MS (PPMS), have very limited treatment options.

Fierce Pharma
NOVEMBER 30, 2022
Regeneron's Evkeeza scores speedy FDA review in bid to treat kids with rare cholesterol disorder. kdunleavy. Wed, 11/30/2022 - 10:06.

Pharmaceutical Technology
NOVEMBER 30, 2022
In what’s being called a “medical bypass”, new anti-obesity drugs are almost matching efficacy rates in weight loss that have previously only been seen with weight loss surgeries. In October, the US FDA granted Eli Lilly’s Mounjaro (tirzepatide) a Fast Track designation for its use as a treatment for obesity. The drug was approved to treat type 2 diabetes mellitus in May.
Celeritas
NOVEMBER 30, 2022
In America, the percentage of households that own or adopt animals is rising every year. A survey conducted by the APPA in 2021-22 showed that 70% of households owned a pet. Additionally, as knowledge of potential threats spreads, veterinary appointments are now part of a pet owner’s routine. The demand for and willingness to spend on extensive care, pet health plans, etc. are also on the rise.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Pharmaceutical Technology
NOVEMBER 30, 2022
Gilead Sciences has received the European Commission’s (EC) expanded marketing authorisation for Biktarvy (bictegravir 30mg / emtricitabine 120mg / tenofovir alafenamide 15mg tablets) for treating human immunodeficiency virus (HIV) infection in virologically suppressed children. The low-dose Biktarvy tablet is indicated for usage in these children aged a minimum of two years and weighing at least 14kg.

Star OUTiCO
NOVEMBER 30, 2022
The tail-end of the year is the time for resolutions and plans for the months to come, and if for you this includes a new job, then you may be able to get ahead of the game by exploring this sooner rather than later. January is one of the busiest recruiting times, so starting your hunt this month could mean lower competition and possibly improve your odds of securing your dream job.
MedCity News
NOVEMBER 30, 2022
Eisai has presented and published full data from the Phase 3 clinical trial for its Alzheimer’s disease drug lecanemab, with results showing a statistically significant slowing decline associated with the neurodegenerative disorder. While an accelerated approval decision is expected in early 2023, the latest trial data are key because they represent the confirmatory study that could support an application for full FDA approval.
pharmaphorum
NOVEMBER 30, 2022
Veeva Systems invited pharmaphorum to attend its industry summit in Madrid this week, a huge event that gathered together over 1,000 individuals from life sciences and pharma. The Marriot Auditorium & Conference Centre was the venue for this occasion, the largest global conference of its kind in Europe, the titular space made clear just how ‘big’ these systems are in facilitating what pharma does, today and very much tomorrow.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

MedCity News
NOVEMBER 30, 2022
Covid-19 has forced us to face inequities present in health outcomes head-on and to recognize the systemic nature of health inequality that goes beyond the limits of what the healthcare system alone can change. The Quintuple Aim is a new framework for addressing these health inequities and centering our innovation and improvement efforts on marginalized communities and populations.

European Pharmaceutical Review
NOVEMBER 30, 2022
Full study results on Eisai’s pivotal Phase III Alzheimer’s (AD) trial for lecanemab, an anti-amyloid beta (A?) protofibril antibody , suggest that the drug could slow the rate of disease progression by 2.5-3.1 years and help people remain in the earlier stages of the disease for longer. The data has been published in The New England Journal of Medicine (NEJM) and was presented at the 2022 Clinical Trials on Alzheimer’s Disease (CTAD) conference in the US.

MedCity News
NOVEMBER 30, 2022
Many of the efforts to address healthcare’s burnout crisis have centered on money, such as hiring and retention bonuses, but that isn’t sustainable. Providers don’t have the means to keep throwing money at the problem forever, so they should focus more on creating a workplace culture where employees feel valued and supported.

Pharma Pathway
NOVEMBER 30, 2022
Ipca Laboratories Ltd- Walk-In Interviews for Formulation Development/ Analytical Development On 10th Dec’ 2022. Job Description. Walk-In Interviews for Formulation Development/ Analytical Development On 10th Dec’ 2022 @ Ipca Laboratories Ltd. Formulation Development. Desired Profile. Candidates with 2-8 years of experience into product development.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

MedCity News
NOVEMBER 30, 2022
While many will remember the Covid-19 pandemic as a source of disruption, it is likely that it will also be viewed as a catalyst for the transformation of medical education that had been brewing for the past decade.

Pharma Pathway
NOVEMBER 30, 2022
Exemed Pharmaceuticals-Openings for B.Pharm/ M.Pharm Freshers- Apply Now. Job Description. Exemed is Vadodara, Gujarat based venture being initiated by a business group involved in Manufacturing of Drug-Intermediates & Specialty Chemicals, for more than three decades, having a reputation for high quality & reliability. Hiring B.Pharm/ M.Pharm Freshers for Regulatory Affairs Department @ Vadodara.
MedCity News
NOVEMBER 30, 2022
Medical device manufacturer iCAD entered into a development and commercialization agreement with Google Health, in which it will integrate Google’s mammography AI technology into its suite of breast imaging AI products. The agreement marks Google’s first commercial partnership to deploy its breast imaging AI model into clinical practice.

Copyright Clearance Center
NOVEMBER 30, 2022
The post Exploring Challenges in the Shift to Open Access: Part 3 – Communication and Collaboration appeared first on Copyright Clearance Center.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

MedCity News
NOVEMBER 30, 2022
Some industry experts seem skeptical of the rumored deal between Amwell and Talkspace, while others disagree. The rumored purchasing price is $200 million at about $1.50 per share.
European Pharmaceutical Review
NOVEMBER 30, 2022
The European Commission (EC) has granted the first paediatric marketing authorisation for Gilead’s new low-dose tablet Biktarvy ® (bictegravir 30mg/emtricitabine 120mg/tenofovir alafenamide 15mg) in the European Union (EU) to treat human immunodeficiency virus (HIV). The EC has authorised extension for Biktarvy ® to treat HIV-infected children over two years old and weighing at least 14kg.
MedCity News
NOVEMBER 30, 2022
As the healthcare industry prepares for a possible recession, Ash Shehata of KPMG expects to see more use of technology, targeted investments and an impact on consumers’ wallets.
European Pharmaceutical Review
NOVEMBER 30, 2022
The UK-based Centre for Process Innovation (CPI) has opened its £88 million Medicines Manufacturing Innovation Centre in Glasgow, Scotland, to help accelerate medicine development by focusing on small molecule and fine chemical manufacturing and sustainable manufacturing. CPI forecasts that the centre will generate £200 million in advanced technologies over the first 5 years, creating over 100 high-value jobs.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

MedCity News
NOVEMBER 30, 2022
Miruna Sasu, CEO of RWE company COTA, explained that studies have shown meaningful improvements in efficiency if RWE is used in drug development and that translates into real savings. However, will such efforts continue amidst the ongoing belt-tightening in a tough economy?
PharmaVoice
NOVEMBER 30, 2022
Methadone, rock ‘n’ roll, clinical trial overhauls — here’s a look at our most-read stories of the year.

PharmaTimes
NOVEMBER 30, 2022
Phase 3 research shows that Alzheimer’s disease treatment meets primary and secondary endpoints

pharmaphorum
NOVEMBER 30, 2022
Twitter has announced that it has ceased enforcing its COVID-19 misinformation policy that prohibits misleading information about SARS-CoV-2. Effective as of 23 November, according to the company website , Twitter will no longer take action against tweets which breach its COVID rules. The news comes not many months after the company revealed that it had suspended more than 11,000 accounts for COVID misinformation that breached its ‘five-strike’ policy.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content