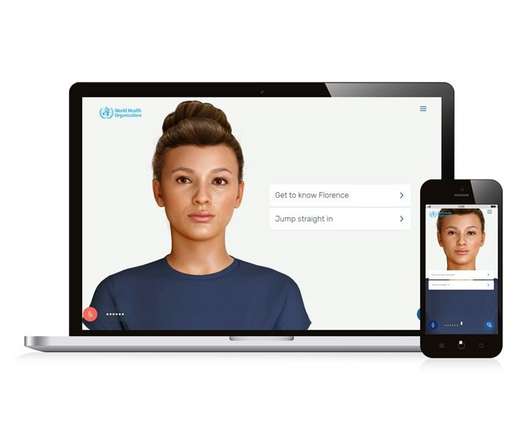
Meet Florence, WHO’s AI-powered digital health worker
pharmaphorum
OCTOBER 6, 2022
An artificial intelligence-powered digital health worker has been unveiled by the World Health Organisation (WHO) as its latest tool for disseminating reliable health information to the public. Originally developed by New Zealand tech company Soul Machines with support of the Qatar Ministry of Health, the first version of the virtual health worker was used to combat misinformation about the pandemic.




































Let's personalize your content