3D Bioprinted Breast Tumors for Immunotherapy Testing
Medgadget
OCTOBER 27, 2022
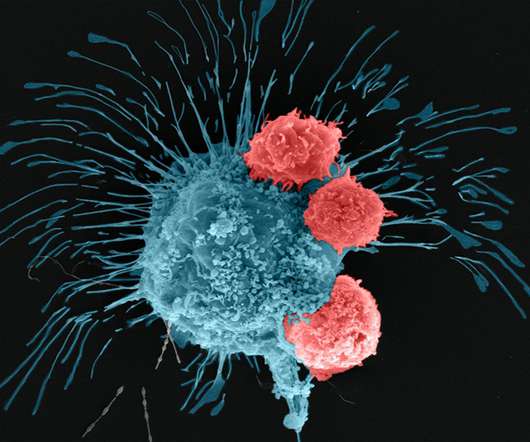
A team of scientists at Penn State has bioprinted breast tumor mimics with significant complexity, including a form of vascularization and the ability to precisely place cells in certain locations within the construct. The scientists used a technique called aspiration-assisted bioprinting to achieve this. With many anti-cancer therapies failing at the clinical trial stage and the ethical considerations of animal studies, there is a need for better in vitro cancer models that allow for advanced t






































Let's personalize your content