5 years after FDA approval, CAR-T treatment looks like the future of medicine
MedCity News
OCTOBER 28, 2022
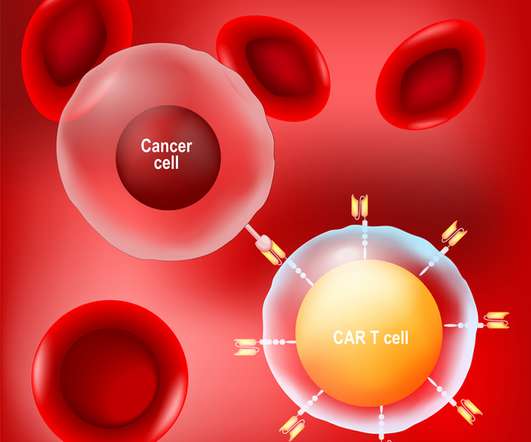
CAR-T has become increasingly recognized as an exciting and potentially paradigm-shifting treatment in the past five years. In fact, more than 10,000 patients have undergone this new treatment for certain types of leukemia, lymphoma. and multiple myeloma.









































Let's personalize your content