Growth in Cell and Gene Therapy Market
PharmaTech
OCTOBER 3, 2022
Biopharma focuses on streamlining biomanufacturing and supply chain issues to drive uptake of cell and gene therapies.

PharmaTech
OCTOBER 3, 2022
Biopharma focuses on streamlining biomanufacturing and supply chain issues to drive uptake of cell and gene therapies.

pharmaphorum
OCTOBER 3, 2022
Gaurav Kapoor, co-founder and executive vice president of Indegene, tells pharmaphorum how pharma can take lessons from the entertainment industry and prepare for the Metaverse to enhance content engagement. Pharma companies’ customer communication methods require reimagining as technology progresses. Kapoor says, now, pharma can learn from the on-demand nature of content distribution that Hollywood utilises and simultaneously prepare for future needs in content delivery. “One of our
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
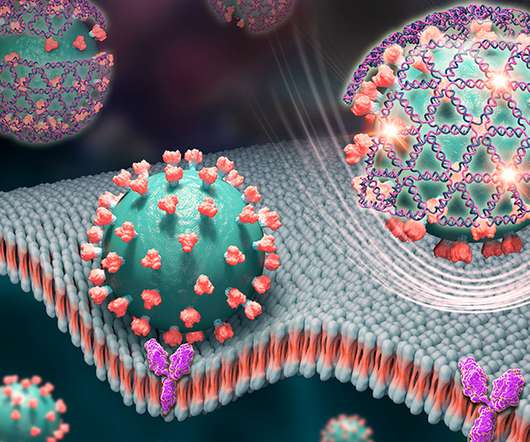
Medgadget
OCTOBER 3, 2022
A team at the University of Illinois at Urbana-Champaign has developed a DNA net system that can ensnare Sars-CoV-2 and bind to the notorious spike protein. The nets contain aptamers that bind the spike protein and emit an intense fluorescent signal once they’re bound together to the protein. This signal can be easily measured using a handheld fluorimeter.
Pharmaceutical Technology
OCTOBER 3, 2022
The UK National Institute for Health and Care Excellence (NICE) has recommended zanubrutinib (Brukinsa) as an option to treat Waldenstrom’s macroglobulinaemia (WM) patients. With the latest development, zanubrutinib became the first WM drug to receive the recommendation for routine National Health Service (NHS) usage in England. The recommendation will also be applicable in Wales and Northern Ireland.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Medgadget
OCTOBER 3, 2022
At the University of Minnesota, researchers have developed a soft robotic system that can ‘grow’ like a plant. The mechanism allows it to travel through difficult-to-access areas, such as the tortuous gastrointestinal tract or vasculature. The system works by extruding a liquid through an opening in the device, and at the same time a photopolymerization process results in the rapid solidification of the liquid into a solid structure.

PharmaTech
OCTOBER 3, 2022
A blended approach to newly revised regulatory guidance to inform environmental monitoring programmes is essential.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

PharmaTech
OCTOBER 3, 2022
Awareness of recently implemented—or ongoing—advances by the pharmacopoeias can help biotherapeutic manufacturers remain compliant with current requirements.

MedCity News
OCTOBER 3, 2022
The digital pathology market is poised for rapid growth in the U.S., with several vendors vying for dominance. The imaging management systems made by Leica Biosystems and Philips seem to be leading the pack in this competitive market, according to a new report.

pharmaphorum
OCTOBER 3, 2022
In draft guidance published on Friday, it emerged NICE does not recommend PTC Therapeutics’ Translarna (ataluren) for the treatment of Duchenne muscular dystrophy (DMD) caused by a nonsense mutation. The decision came following a re-evaluation of Translarna in order to decide whether to make it available for routine funding on the NHS. The re-evaluation followed a six-year period during which the DMD drug had been available under a managed access agreement (MAA), which ends in January next year,

PharmaTech
OCTOBER 3, 2022
Various advances in contamination control are being utilized to reduce the chance pollutants contaminate a drug product.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

MedCity News
OCTOBER 3, 2022
Innovative approaches to real world evidence collection present opportunities for medical device companies to improve patient outcomes. But success will depend on improving the way medtech companies integrate digital health. Executives from Abbott, Smith & Nephew, AliveCor and Huma weigh in on these issues.

PharmaTech
OCTOBER 3, 2022
External expertise is beneficial in helping companies select the right tools at the right stages of development to ensure success.

PharmaVoice
OCTOBER 3, 2022
In the past, patients entrusted their healthcare providers to provide information about health-related matters.

PharmaTech
OCTOBER 3, 2022
Whether biologic manufacturers decide to outsource or develop products internally, the quality of a CDMO partnership is critical to success, especially for cell and gene therapy products.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

PharmaTimes
OCTOBER 3, 2022
Events will raise awareness around the impact of atopic dermatitis across cultures
PharmaTech
OCTOBER 3, 2022
BFS technology can help maintain sterility during the biologics manufacturing process.

PharmaTimes
OCTOBER 3, 2022
The anti-inflammatory treatment is for Duchenne muscular dystrophy

pharmaphorum
OCTOBER 3, 2022
Biotech company Zealand Pharma A/S, an innovator in peptide-based medicines, announced the positive topline results from its phase 3 trial of glepaglutide on Friday. A total of 106 patients with short-bowel syndrome (SBS) who also experienced intestinal failure and dependency on parenteral support (PS) at least three days per week took part in the evenly randomized double-blind trial, receiving treatment with 10mg glepaglutide either once or twice weekly, or a placebo.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Healthcare Success
OCTOBER 3, 2022
Google Search is always updating its algorithm, especially when it comes to content. As an agency, we keep track of these updates and make sure our clients’ websites comply with the latest search engine standards. On August 25 of this year, the search giant rolled out yet another update, this time dubbed the “helpful content update.” The goal of this signal is to reward high-quality content while also penalizing websites with content created just for SEO.

MedReps
OCTOBER 3, 2022
Among the many specialties in the medical sales field are medical devices. This particular niche is one chosen by numerous people due to its compensation and benefits, not to mention the amazing and life-saving devices themselves. Many people prefer selling these medical devices over other options, such as biotech products and pharmaceuticals. Why, you ask?

Scott’s Directories
OCTOBER 3, 2022
Geo-targeting is the practice of delivering content to people based on their geo-locations. This includes countries, states, cities, zip codes, IP addresses, and more. Geo-targeting is often used for online advertising and promotions to reach local prospects through paid search campaigns. For instance, Google Ads geotargeting allows advertisers to specify a location as the only area where they want their ads to show.

PharmaVoice
OCTOBER 3, 2022
Long-term results showed five-year survival numbers for lung cancer patients that surpassed all others, and Merck's VP of oncology research tells us what he's excited about.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

Pharma Pathway
OCTOBER 3, 2022
Aishwarya Healthcare – Urgent requirements for Accounts / Corporate HR/QC/ Engineering Dept. -Apply Now. Job Description. Opening for Accounts / Corporate HR/QC/ Engineering Departments @ Aishwarya healthcare . Job Posted Date: 03/10 /2022. Last Date: 08/10/ 2022. Accounts department – Job opening for Sr.Executive/ Assistant manager with 6 to 8 years of experience in Cost accounts and accounting distributions section.
The Marketing Advantage
OCTOBER 3, 2022
October 3, 2022 With 2023 sales compensation planning underway, many biopharma companies are considering whether or not they should change their sales compensation plan and, if so, how much. A sales compensation plan that is not motivating the sales force as well as it could be is ultimately leaving sales on the table, and there are several indicators that companies can look to in order to help determine if it is time to change their sales compensation plan.

PharmExec
OCTOBER 3, 2022
November 29th 2022 9 am CT |10 am ET | 3 pm BST | 4 pm CET Statistical signal detection is a crucial tool for rapidly identifying potential risks associated with pharmaceutical products. New methods for signal detection offer the potential to identify adverse reactions from vaccines and drugs earlier.
Pharmaceutical Technology
OCTOBER 3, 2022
Myovant Sciences has rejected an acquisition offer worth $2.5bn from Sumitovant Biopharma and its wholly-owned subsidiary Sumitomo Pharma, citing it 'significantly undervalues' the company. Sumitovant and Sumitomo submitted a non-binding proposal to the audit committee of the board of directors of Myovant for the acquisition of all outstanding shares of the company that are not currently held by Sumitovant for $22.75 for each share in cash.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
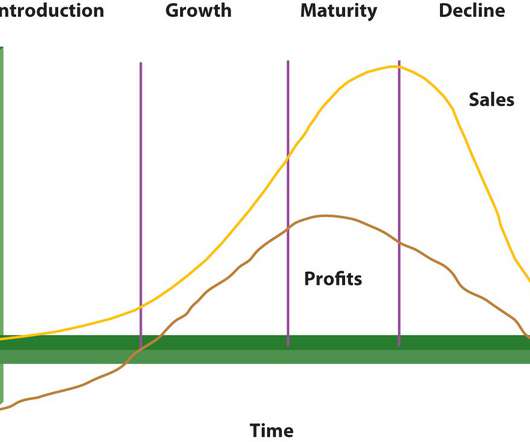
eMediWrite
OCTOBER 3, 2022
All products and services have certain life cycles. The term “life cycle” describes the time span between a product’s first release onto the market and its eventual withdrawal. Significant adjustments are made to the product’s market behavior during this time, which is reflected in the sales to the firm that brought it to the market.

Pharmaceutical Technology
OCTOBER 3, 2022
Indonesia has granted Emergency Use Authorization (EUA) for the patent-free Covid-19 vaccine, IndoVac, developed by the Texas Children’s Hospital Center for Vaccine Development (CVD) and Baylor College of Medicine, US. PT Bio Farma will manufacture 20 million doses of the vaccine this year. Additionally, the company will produce another 100 million vaccine doses by 2024.

eMediWrite
OCTOBER 3, 2022
Brand awareness, used in marketing, refers to how well customers can name a product. Customers’ awareness of the brand should ideally include favorable opinions about the characteristics that set the product apart from its rivals. Promotion of a new product or reinvigoration of an established brand both needs building brand awareness. These are some points you can use to build brand awareness: Target specific audiences with your branding efforts.

pharmaphorum
OCTOBER 3, 2022
Amylyx Pharmaceuticals’ Relyvrio – a new treatment for amyotrophic lateral sclerosis (ALS) approved by the FDA on Thursday, after review of the data from its phase 2 trial – was the next day set at a list price of $158,000 per year in the US, sparking outcry. The third ALS treatment to be given FDA approval, following Mitsubishi Tanabe’s Radicava ($170,000 per year) and generic drug riluzole – Relyvrio is priced at around $12,500 per 28-day prescription.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content