With buyout on Horizon, Amgen and Sanofi eye cash plays for rare disease drug maker
Fierce Pharma
DECEMBER 2, 2022
With buyout on Horizon, Amgen and Sanofi eye cash plays for rare disease drug maker. fkansteiner. Fri, 12/02/2022 - 09:51.

Fierce Pharma
DECEMBER 2, 2022
With buyout on Horizon, Amgen and Sanofi eye cash plays for rare disease drug maker. fkansteiner. Fri, 12/02/2022 - 09:51.

MedCity News
DECEMBER 2, 2022
From streamlining long-held manual processes to creating entirely new diagnostic devices, new tech is cropping up all over the medtech industry—resulting in a real-world impact on patients’ quality of life.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
DECEMBER 2, 2022
UPDATED: GSK, with new trial win, looks to expand PD-1 Jemperli in endometrial cancer. zbecker. Fri, 12/02/2022 - 11:22.

MedCity News
DECEMBER 2, 2022
Health system leaders believe that improving patient access should be their top priority when strategically planning for 2023, according to a new report from the KLAS Research and UPMC’s Center for Connected Medicine. To improve patient access, respondents agreed that their health systems will need to make changes in three key areas — people, process and technology.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Fierce Pharma
DECEMBER 2, 2022
Fierce Pharma Asia—Eisai's lecanemab Alzheimer's debate; BeiGene's Imbruvica showdown; Shionogi's COVID drug nod. aliu. Fri, 12/02/2022 - 08:55.

MedCity News
DECEMBER 2, 2022
Lumen sells a handheld device that measures users’ metabolism through breath and connects to an app that helps track goals and provides nutrition coaching. The funding round was led by Pitango Venture Capital, and included participation from Hanwha Group and Resolute Ventures.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

MedCity News
DECEMBER 2, 2022
The lawsuit is one of nine cases throughout the country that TeamHealth has brought against United for alleged underpayments. The other eight cases are still ongoing. .
Fierce Pharma
DECEMBER 2, 2022
Rigel scores green light in AML just 4 months after buying asset from Forma. kdunleavy. Fri, 12/02/2022 - 10:00.

MedCity News
DECEMBER 2, 2022
FDA’s draft guidances on real-world data do not provide strict requirements for industry stakeholders to abide by, but they offer patient advocacy organizations with a clearer understanding of how to model their registries to have a more substantial impact for their patient communities.

PharmaTech
DECEMBER 2, 2022
QMM is an evolutionary advancement in FDA’s quality management maturity initiative that provides industry with a viable methodology to assess and improve manufacturing quality and supply chain reliability.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Medico Reach
DECEMBER 2, 2022
To bring effective medicines and Innovations in healthcare industry , a Clinical Research Organization or Contract Research Organization (CRO) are a must for the Biotech, Medtech, and pharma industries. A CRO offers comprehensive support to their efforts to test, examine, refine and market the latest medicines and devices that help in improving Healthcare Industry.

pharmaphorum
DECEMBER 2, 2022
Enrolling for clinical trials has become more challenging in recent years, with trial competition, increasingly complex protocols, and precision medicine targeting more niche patient populations. This emphasises sponsors’ need for enrolment predictability. The challenge the industry faces, however, is that operational certainty is exacerbated by the impact of global disruptions, such as the effect of COVID-19, natural disasters, and regional conflicts.

MedCity News
DECEMBER 2, 2022
Check out new developments from Medvantx, Medisafe, Medical Informatics Corp., Journey Biosciences, and GSR Ventures.
European Pharmaceutical Review
DECEMBER 2, 2022
Vaccines Europe has unveiled its first pipeline review of its 15 members companies, with 100 vaccine candidates as of July 2022. . The review revealed a pipeline aimed at tackling current and future challenges, such as antimicrobial resistance (AMR) and respiratory tract infections, through leveraging a wide range of new technology platforms. The data indicated 46 percent of the vaccine candidates target infections that do not have an available vaccine.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharma Pathway
DECEMBER 2, 2022
Intas Pharmaceuticals Ltd-Walk-In Drive for M.Sc (Microbiology) in Quality Control On 7th Dec’ 2022. Job Description. Company Profile: INTAS Pharmaceutical is a leading, vertically integrated global pharmaceutical formulation development, manufacturing, and marketing company. Intas is committed to challenging the unmet medical and societal needs through a comprehensive pharmaceutical value chain spanning across the world.

European Pharmaceutical Review
DECEMBER 2, 2022
Pfizer Inc. has announced it will invest over €1.2 billion in its manufacturing site in Grange Castle , Dublin, the company’s biggest expansion investment to date in Ireland. A new facility will be built on the site premises, doubling the capacity for biologics manufacturing at the facility. It is anticipated a further 400-500 roles will be created, bringing the total Pfizer employees in Ireland to around 5,500.

Pharmaceutical Technology
DECEMBER 2, 2022
India’s Central Drugs Standard Control Organisation (CDSCO) has granted approval for Bharat Biotech’s BBV154 vaccine for treating Covid-19. BBV154 is claimed to be the first intra-nasal vaccine for Covid-19 in the world. It has been approved for restricted use in emergencies for people aged 18 and above in India for the first two-dose schedule and homologous booster doses.

European Pharmaceutical Review
DECEMBER 2, 2022
Rigel Pharmaceuticals, Inc. has announced the US Food and Drug Administration (FDA) has approved its oral drug Rezlidhia (olutasidenib) for adults with relapsed or refractory (R/R) acute myeloid leukaemia (AML) with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation. The FDA approval was supported by open-label Phase II registrational study data, which showed a twice per day 150mg dose of Rezlidhia facilitated a complete remission (CR) + CRh rate of 35 percent of the 153 patients observed.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

PharmaVoice
DECEMBER 2, 2022
The metaverse, blockchain, DCTs and more — here are some of the most recent fails in healthcare tech.

Copyright Clearance Center
DECEMBER 2, 2022
The post OA Agreement Intelligence: A Collaborative Approach to Innovation appeared first on Copyright Clearance Center.
pharmaphorum
DECEMBER 2, 2022
A new global prostate cancer awareness campaign developed by biopharmaceutical companies AstraZeneca (AZ) and Merck & Co. (MSD) has just been launched in the US and Canada: ‘Never Miss’ leverages sport – as well as social media activity and an information website – as a way to help men understand their potential risk of developing prostate cancer, opening up the conversation about this sensitive topic: Never miss a game.

Copyright Clearance Center
DECEMBER 2, 2022
The post HarperCollins Union Members On Strike appeared first on Copyright Clearance Center.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

PharmaTimes
DECEMBER 2, 2022
Patients and service users routinely struggle to access mental healthcare across the NHS

PharmaTech
DECEMBER 2, 2022
Unique solutions are required to protect inherently unstable messenger RNA.

PharmaTimes
DECEMBER 2, 2022
Positive scientific opinion by EMA would create pathway for arpraziquantel in African endemic countries

pharmaphorum
DECEMBER 2, 2022
Following the news of two lecanemab-related deaths reported on the eve of the Clinical Trials on Alzheimer’s Disease (CTAD) 2022 conference, Eisai has now presented the full results from its phase 3 confirmatory Clarity AD study for early Alzheimer’s disease and published them in the New England Journal of Medicine (NEJM). The full result from Eisai’s large, global phase 3 confirmatory Clarity AD clinical study of lecanemab – an investigational anti-amyloid beta (A?

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Cesare Ferrari
DECEMBER 2, 2022
This is the second post about upselling and cross selling where I’ll be talking about how to effectively implement these two often misunderstood tactics. You can check part 1 here to understand the basics. How to effectively upsell and cross sell. 1. Keep customer focus. Active listening is one of the most important skills of an effective sales rep.
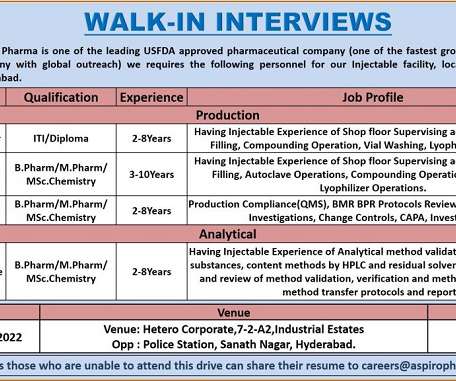
Pharma Pathway
DECEMBER 2, 2022
Aspiro Pharma -Walk-In Interviews for Production/ Analytical On 4th Dec’ 2022. Job Description. Greetings from Aspiro Pharma !!! Walk-In Interviews for M.Sc/ B.Pharm/M.Pharm/ ITI/ Diploma in Production/ Analytical Department @ Aspiro Pharma . Department: Production/ Analytical. Position: Officer – Sr. Executive/ Operator-Sr. Operator. Experience: 02 to 10 years.

PharmExec
DECEMBER 2, 2022
Agility and better decisions depend on managing risk and gaining insights from data by prioritizing patient and end-user needs. Companies of all sizes, including GSK, Boehringer-Ingelheim, Sanofi, AstraZeneca, and AbbVie, share best practices.

Pharma Pathway
DECEMBER 2, 2022
FDC Limited- Openings for Quality Assurance/ Packing Departments-Apply Now. Job Description. Department: Quality Assurance/ Packing. Designation : Officer. Qualification: B.Pharm. Experience: 03 to 05 years. Work Location: Goa. Interested Candidates can Share their CV On anesh.shet@fdcindia.com. FDC Limited- Openings for Quality Assurance/ Packing Departments-Apply Now.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content