Fierce Pharma Asia—China's reimbursement round; Sun's alopecia bet; GenScript's CDMO raise
Fierce Pharma
JANUARY 19, 2023
Fierce Pharma Asia—China's reimbursement round; Sun's alopecia bet; GenScript's CDMO raise aliu Thu, 01/19/2023 - 15:53
Fierce Pharma
JANUARY 19, 2023
Fierce Pharma Asia—China's reimbursement round; Sun's alopecia bet; GenScript's CDMO raise aliu Thu, 01/19/2023 - 15:53
Medgadget
JANUARY 19, 2023
Researchers at Linköping University in Sweden have developed artificial neurons that demonstrate 15 of the 20 characteristics of biological neural cells and can communicate with natural neurons in the body. The researchers call their device the “conductance-based organic electrochemical neuron,” or c-OECN, and it is based on materials that can conduct a negative charge, including organic electrochemical transistors and n-type conducting polymers.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
JANUARY 19, 2023
Roche touts industry-first liver cancer win, expects more Tecentriq adjuvant data this year aliu Thu, 01/19/2023 - 09:17

PharmExec
JANUARY 19, 2023
Fran Pollaro talks with Amanda Powers-Han, chief marketing officer at Greater Than One, a full-experience marketing agency dedicated to healthcare, about what’s trending in early 2023 for pharma media mixes.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
JANUARY 19, 2023
Bluebird extends cash runway to late 2024 with $113M raise through stock sale esagonowsky Thu, 01/19/2023 - 08:30
pharmaphorum
JANUARY 19, 2023
Biotech Vaxxas has announced a partnership agreement with the 2017-established Coalition for Epidemic Preparedness Innovations (CEPI), in order to advance the development of Vaxxas’ needle-free vaccine-patch delivery technology with preclinical testing of an mRNA vaccine patch. CEPI is a partnership between public, private, philanthropic, and civil organisations to develop vaccines against future epidemics and pandemics.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

MedCity News
JANUARY 19, 2023
Eli Lilly’s accelerated approval application for its Alzheimer’s disease drug has fallen short. The company said the FDA is asking for data from more patients—results that must come from a larger study that’s underway but won’t report data until later this year.

Fierce Pharma
JANUARY 19, 2023
Alnylam, PTC Therapeutics rare disease drugs win backing from England's NICE zbecker Thu, 01/19/2023 - 11:53

MedCity News
JANUARY 19, 2023
Women have been dismissed and neglected for centuries: any investment and innovation in this space can make an outsized impact: keeping women and their families healthy, aiding research and development, and eliminating the gender bias against women and their healthcare that has persisted since Ancient Greece.

Fierce Pharma
JANUARY 19, 2023
Amarin, in spat with activist investor, says Sarissa has 'no plan and no new ideas' kdunleavy Thu, 01/19/2023 - 10:11

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Pharmaceutical Technology
JANUARY 19, 2023
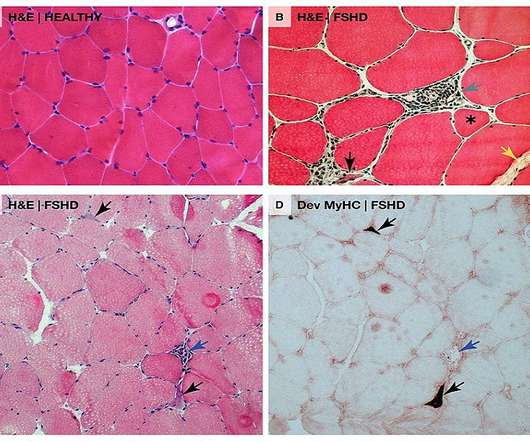
The US Food and Drug Administration (FDA) has granted Fast Track designation for Avidity Biosciences’ AOC 1020 to treat facioscapulohumeral muscular dystrophy (FSHD). AOC 1020 has been designed for the treatment of the underlying cause of FSHD, which is caused by the abnormal expression of a gene known as double homeobox 4 or DUX4. This DUX4 protein abnormal expression leads to modifications in gene expression in muscle cells which are associated with progressive muscle function loss in FSHD pat

Fierce Pharma
JANUARY 19, 2023
Is the first NASH drug approval nearing?

Storyvine
JANUARY 19, 2023
As a sales representative, connecting with healthcare professionals (HCPs) is an important part of your job. There are many tools you can use to make this process more effective and efficient, but one that is often overlooked is video. Let's take a look at the reasons why Sales Reps should add video to their toolbox: 1. Video is a powerful tool that can help you build trust and relationships with HCPs.

Fierce Pharma
JANUARY 19, 2023
Seagen's targeted cancer med Tukysa picks up accelerated FDA nod in colorectal cancer esagonowsky Thu, 01/19/2023 - 15:39

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

MedCity News
JANUARY 19, 2023
Point32Health will now be able offer members access to LGBTQ+ Health, Included Health’s navigation and advocacy program for LGBTQ+ members. These members will have access to a care team led by LGBTQ+ care coordinators, who can help them find in-network, gender-affirming providers. They can also be connected to support groups and receive education on medical and legal issues.
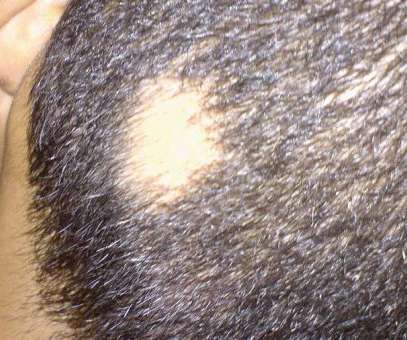
pharmaphorum
JANUARY 19, 2023
India’s Sun Pharmaceutical has signed an agreement to acquire US biotech Concert Pharmaceuticals and its lead drug deuroxilitinib, in phase 3 testing for alopecia areata, a leading cause of hair loss. The $11.50 per share deal includes $8 per share upfront, worth around $576 million, with the remainder in the form of a contingent value right (CVR) that will be paid to Concert shareholders if deuroxilitinib meets certain sales targets within a set time limit.
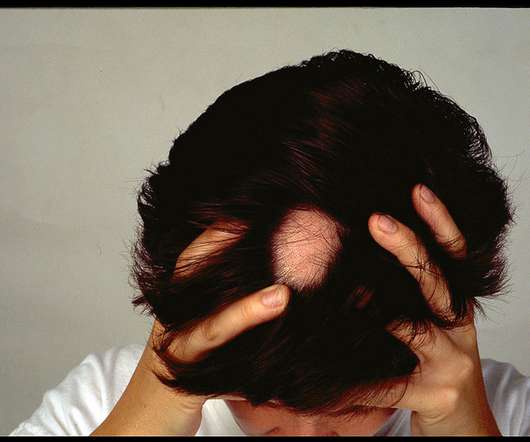
MedCity News
JANUARY 19, 2023
Sun Pharmaceuticals is acquiring Concert Pharmaceuticals, a biotech whose lead program has positive data from two late-stage clinical trials in alopecia areata. The deal gives Sun Pharma a drug candidate that could match up against hair loss drugs from two big pharmaceutical companies.
European Pharmaceutical Review
JANUARY 19, 2023
Tecentriq ® (atezolizumab) plus Avastin ® (bevacizumab) improved recurrence-free survival (RFS) when compared to active surveillance, in Genentech’s Phase III trial for early-stage hepatocellular carcinoma (HCC). “ IMbrave050 is the first Phase III study to show that a cancer immunotherapy combination reduced the risk of disease returning in people with early-stage HCC,” commented Dr Levi Garraway, PhD, Chief Medical Officer and Head of Global Product Development at Roche.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
MedCity News
JANUARY 19, 2023
Posterity Health recently raised $7.5 million in an oversubscribed funding round. The startup’s digital male fertility platform offers a combination of virtual visits, at-home diagnostics and in-person consultations to assess and improve patients’ fertility status.

pharmaphorum
JANUARY 19, 2023
On the sidelines of JP Morgan 2023, pharmaphorum editor-in-chief Jonah Comstock sat down with Raj Chopra, acting CEO of Aethon Therapeutics. Aethon recently launched January 9th, with $30 million in funding as a collaboration between Apple Tree Partners and NYU Langone Health. In this interview, Chopra describes the HapImmune technology behind the company, which uses customised antibodies to create covalent inhibitors that bind to target proteins to create a peptide conjugate “beacon” that only

MedCity News
JANUARY 19, 2023
Abbott recently earned FDA approval for its latest transcatheter aortic valve implantation (TAVI) system, which is named Navitor. Abbott will have to fight for its place the U.S. TAVI device space, as the systems made by Edwards and Medtronic have a steadfast command of the market share.

PharmaVoice
JANUARY 19, 2023
The U.S. agency’s lower number of novel drug approvals followed several controversies surrounding a 2021 Alzheimer’s approval.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
European Pharmaceutical Review
JANUARY 19, 2023
Over the next four years, Amgen has committed to contribute $8 million to the Robert A. Winn Diversity in Clinical Trials Award Program ( Winn Award Program ), established by the Bristol Myers Squibb Foundation (BMSF). The contribution will enable the programme to reach additional physicians and medical students, further expanding the initiative that aims to extend the reach of clinical trials in underserved patient populations in US communities.

pharmaphorum
JANUARY 19, 2023
With a new year comes an opportunity for professionals in the healthcare industry to evaluate goals and initiatives for 2023. In business, we’re constantly reminded that with even the best-laid plans there come unforeseen obstacles. In this article, I’ll outline some of the challenges that I anticipate for 2023. At Skyscape, we’re developing mobile solutions for the modern healthcare professional to help ameliorate these challenges, and in the spirit of our mission I offer advice to organisation

PharmaTimes
JANUARY 19, 2023
Therapy is among the first to receive a positive draft recommendation under streamlined pilot process

pharmaphorum
JANUARY 19, 2023
Run by Life Science Integrates, Pharma Integrates was in its 11th year in 2022. It was a unique event, at which leaders from across the pharmaceutical pipeline addressed the needs of the industry, shared their own insights, and tackled debates on the crucial topics that influence the future of patient outcomes. With pharmaphorum in attendance, the day continued from the morning’s welcome from Trevor Jones, chairman of e-Therapeutic Plc., from the introduction by Susan Rienow, country president U

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

PharmaTech
JANUARY 19, 2023
It’s crucial to consider the optimal handling systems for cleanroom and other lab environments.
European Pharmaceutical Review
JANUARY 19, 2023
The National Institute for Health and Care Excellence (NICE) has recommended Ninlaro ® (ixazomib) with lenalidomide and dexamethasone for treating relapsed/refractory multiple myeloma (RRMM). The final appraisal document (FAD) recommended the triple regimen as an option for adult patients who have received two or three lines of therapy. Shelagh McKinlay, Head of Patient Advocacy at Myeloma UK stated: “There aren’t many treatment options for patients in the third-line setting… This regimen also p

MedCity News
JANUARY 19, 2023
History has shown that while healthcare is more open to adopting technology to improve outcomes, […]

PharmaTimes
JANUARY 19, 2023
The new funding package will be used to progress NeoPhore’s expanding pipeline of therapies

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content