Eli Lilly dumps Innovent's PD-1 after FDA rebuff, nixing high-profile Chinese cancer drug
Fierce Pharma
DECEMBER 1, 2022
Eli Lilly dumps Innovent's PD-1 after FDA rebuff, nixing high-profile Chinese cancer drug. aliu. Thu, 12/01/2022 - 15:03.

Fierce Pharma
DECEMBER 1, 2022
Eli Lilly dumps Innovent's PD-1 after FDA rebuff, nixing high-profile Chinese cancer drug. aliu. Thu, 12/01/2022 - 15:03.
MedCity News
DECEMBER 1, 2022
Thanks to the convergence of cloud technologies, AI, and refined lung data, we are in a new era for those with chronic lung disease. In this new world, lung intelligence provides fast, precision measures of treatment efficacy so new drugs can blaze through the trial pipeline.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
DECEMBER 1, 2022
With FDA nod for its fecal microbiome therapy, Ferring becomes No. 1 in No. 2. kdunleavy. Thu, 12/01/2022 - 10:14.

MedCity News
DECEMBER 1, 2022
An AHIP survey found that 73% of commercially-insured telehealth users think that Congress should make telehealth provisions permanent. These provisions were put in place to meet Americans’ needs during Covid-19.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
DECEMBER 1, 2022
Roche, having shared data with regulators, unwraps results of subcutaneous Tecentriq phase 3 trial. ntaylor. Thu, 12/01/2022 - 09:45.

pharmaphorum
DECEMBER 1, 2022
Lack of access, strict regulations, and demanding schedules have made it extremely difficult for patients to participate in clinical trials. A 2018 NIH survey found that patients felt clinical trial participation to be inconvenient and burdensome, and nearly half (49.0%) said it disrupted their daily routine. In 2021, a CISCRIP Perceptions and Insights Study reported more disruption to daily routines compared to previous years, citing length of visits, travel, and diagnostic tests as top burdens
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

MedCity News
DECEMBER 1, 2022
UpStream — a company that provides technology and services for physicians enrolled in value-based care models — raised $140 million in Series B funding. The company doesn’t charge providers to use its services. It has negotiated rates with Medicare programs and takes on both upside and downside risk — but physicians “only see the upside” because they get paid by UpStream no matter what, according to CEO Sanjay Doddamani.

Fierce Pharma
DECEMBER 1, 2022
Persistent Lexicon still working to get an audience with FDA on Type 1 diabetes prospect. kdunleavy. Thu, 12/01/2022 - 07:06.

MedCity News
DECEMBER 1, 2022
Medical software company Cerner must head to trial for a jury to decide whether its software contained design defects that caused brain damage to a 25-year-old who was undergoing surgery to remove his gallbladder. .

Fierce Pharma
DECEMBER 1, 2022
Regeneron's Libtayo posts lung cancer win with Bristol's Yervoy. But company won't seek approval. aliu. Thu, 12/01/2022 - 10:15.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

MedCity News
DECEMBER 1, 2022
The innovation institute run by the University of North Carolina at Chapel Hill teamed up with Amazon Web Services to create a venture studio to turn the university’s digital health research concepts into full-fledged startups. AWS’ platform beat out the other major cloud providers because it was “the most entrepreneurial,” according to the venture studio’s managing director.

Fierce Pharma
DECEMBER 1, 2022
FDA shelves Eli Lilly's COVID antibody bebtelovimab as evasive subvariants take hold. zbecker. Thu, 12/01/2022 - 09:07.

Medgadget
DECEMBER 1, 2022
Bigfoot Biomedical , a medtech company based in California, has developed the Bigfoot Unity System, a diabetes management technology for patients on multiple daily injection therapy. The system uses continuous glucose monitoring data and doctor recommendations to provide insulin dose recommendations, helping patients to avoid uncertainty. The company argues that type 2 diabetes patients have been historically underserved by the medtech industry, in part because such patients typically tend to be

Fierce Pharma
DECEMBER 1, 2022
With J&J's Gorsky departing, Duato adopts dual role as chairman and CEO. zbecker. Thu, 12/01/2022 - 11:20.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

MedCity News
DECEMBER 1, 2022
People are living longer and this growing patient population needs innovative treatments tailored to the needs of aging bodies, which is why clinical research must include elderly patients.
Legacy MEDSearch
DECEMBER 1, 2022
HemoSonics, LLC , a leading medical device company delivering individualized diagnostic solutions for Patient Blood Management (PBM), announced that it has received 510(k) market clearance from the U.S. Food and Drug Administration (FDA) for the Quantra Hemostasis System with QStat Cartridge. “The Quantra Hemostasis System with QStat Cartridge is breaking new ground and leading innovation in the point-of-care and laboratory-based whole blood hemostasis testing market.

MedCity News
DECEMBER 1, 2022
In the third quarter of 2022, BCBS Massachusetts’ members had about 8 million behavioral health visits, compared to about 4 million in 2019. In response, the insurer is expanding its mental health services by contracting with Talkiatry, And Still We Rise and DynamiCare.

pharmaphorum
DECEMBER 1, 2022
In the newest episode of the pharmaphorum podcast, editor in chief Jonah Comstock chats with Gita Barry, EVP and GM of Immersive Healthcare at Penumbra, a therapeutic VR company. Penumbra uses VR to improve the physical therapy experience – both making it more fun for patients and delivering certain types of therapy, like mirror therapy, more effectively than traditional PT methods.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

MedCity News
DECEMBER 1, 2022
In a webinar, flipMD and physicians discussed how the organization connects pioneering healthcare companies with expert physicians to maximize patient impact.

PharmaVoice
DECEMBER 1, 2022
Why South Rampart Pharma’s novel new molecule could be a key to solving the global pain epidemic.
European Pharmaceutical Review
DECEMBER 1, 2022
According to GlobalData’s recent report , a total of five marketed messenger RNA (mRNA) non-vaccine products by 2028 will generate over $2 billion, generated by the approval of pipeline agents. The analytics company asserted that despite no mRNA non-vaccine therapeutics currently being in the market, this statistic is derived from the combined revenue expected from Moderna, Ultragenyx, Omega Therapeutics, and BioNTech’s investigational therapies for cancer and rare genetic diseases, all in

Pharma Pathway
DECEMBER 1, 2022
Endo India Par Formulations-Walk-In Interviews for Quality Control/ Manufacturing On 4th Dec’ 2022. Job Description. Endo India Par Formulations is a pharmaceutical company that develops, manufacturers and markets safe, innovative and cost-effective pharmaceuticals that help improve patient quality of life. At Endo our investment in state-of-the-art equipment and facilities, commitment to ethical standards, and growing portfolio of products makes us a company where you can enjoy a producti

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
European Pharmaceutical Review
DECEMBER 1, 2022
Rebyota, the first faecal microbiota product to prevent recurrence of Clostridioides difficile infection (CDI) in people 18 years and older after being given antibiotics for recurrent CDI, has been approved by the US Food and Drug Administration (FDA). The approval, granted to Ferring Pharmaceuticals Inc, was given based on safety data from two randomised, double-blind, placebo-controlled studies and open-label clinical studies in the US and Canada in participants with a history of one or more C

PharmaVoice
DECEMBER 1, 2022
A potential flurry of regulatory activity could be on the horizon yet this year — and several key drug approvals are at stake.

Copyright Clearance Center
DECEMBER 1, 2022
December 1, 2022 – Danvers, Mass. – CCC , a leader in advancing copyright, accelerating knowledge, and powering innovation, will host its next virtual “ Workflow of the Future ” event on Thursday, 8 December at 11:00 am EST focused on standards and sustainability. Industry experts will discuss how standards help businesses prioritize the environment.
Medgadget
DECEMBER 1, 2022
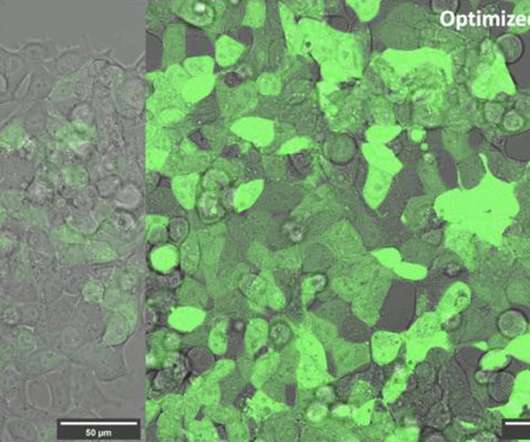
Scientists at Hong Kong University of Science and Technology came up with a technique to increase the efficiency and potentially the efficacy of mRNA therapeutics. mRNA molecules have what is called a poly-A tail, which is basically a string of adenine nucleotides at one end. These researchers discovered that by replacing some of these nucleotides in the mRNA tail with cytidine, a cytosine base with a ribose sugar attached, that they could enhance the resulting protein production of the mRNA and

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Pharma Pathway
DECEMBER 1, 2022
Emcure Pharmaceuticals-Walk-In Interviews for Quality Assurance/ Engineering On 4th Dec’ 2022. Job Description. Emcure Pharmaceuticals, We are a fast-growing Indian Pharmaceutical company engaged in developing, manufacturing, and marketing a broad range of biopharmaceutical products globally. Emcure’s differentiated product portfolio lends and unparalleled competitive advantage establishing its presence in all major therapies in the domestic market.
Map My Customers
DECEMBER 1, 2022
Data lovers, meet location intelligence. Location intelligence is one of the hottest trends in machine learning—and it’s a match made in heaven for outside sales professionals. From opportunity visualization to sales territory management to route optimization — location intelligence is your ticket to moving past spreadsheets and toward geospatial visualizations.

Pharma Pathway
DECEMBER 1, 2022
Medreich Limited -Walk-In Interview for QA/ QC/ Production/ Engineering/ Technology Transfer (TT) On 3rd Dec’ 2022. Job Description. Walk-In Interview for QA/ QC/ Production/ Engineering/ Technology Transfer (TT) For Freshers & Experienced @ Medreich Limited. Department: QA/ QC/ Production/ Engineering/ Technology Transfer (TT). Qualification: B.Pharm/ M.Pharm/ B.E/ B.Tech.
pharmaphorum
DECEMBER 1, 2022
Myeloma Patients Europe (MPE) has identified significant inequalities among patients’ access to myeloma clinical trials in Central and Eastern Europe (CEE) in a first-of-its-kind advocacy report. Led by the MPE CEE Workgroup on Access, the report – Addressing access barriers to myeloma clinical trials in Central and Eastern Europe – evaluated the number of clinical trials held in CEE countries between 1 January 2001 and 28 September 2020.

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content