Sorry, the Inflation Bill won’t lower healthcare costs
World of DTC Marketing
AUGUST 19, 2022
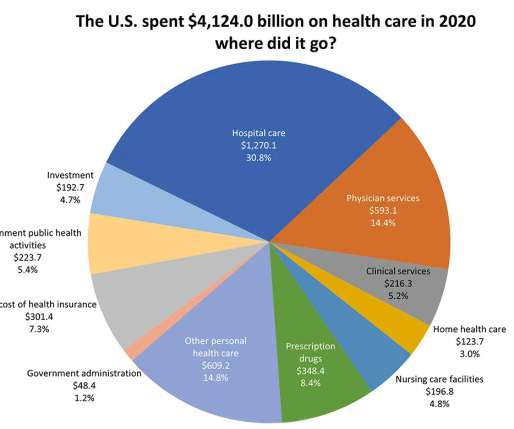
The Inflation Reduction Act’s health insurance subsidies and drug pricing reforms will improve health care affordability for Americans but won’t do a damn thing for our overall healthcare costs, which will keep rising. High drug and health care costs prevent millions of Americans from achieving total health. Nearly 1 in 2 American adults report difficulty affording health care costs.

































Let's personalize your content