When the FDA rejected Intercept Pharmaceuticals’ drug candidate for non-alcoholic steatohepatitis (NASH) two years ago, the agency didn’t ask for another clinical trial. It said it wanted more data from the ongoing Phase 3 study. That study has accrued more safety and efficacy data, and Intercept now says these results support a second shot at seeking FDA approval. A meeting with the regulator is scheduled for later this month.

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
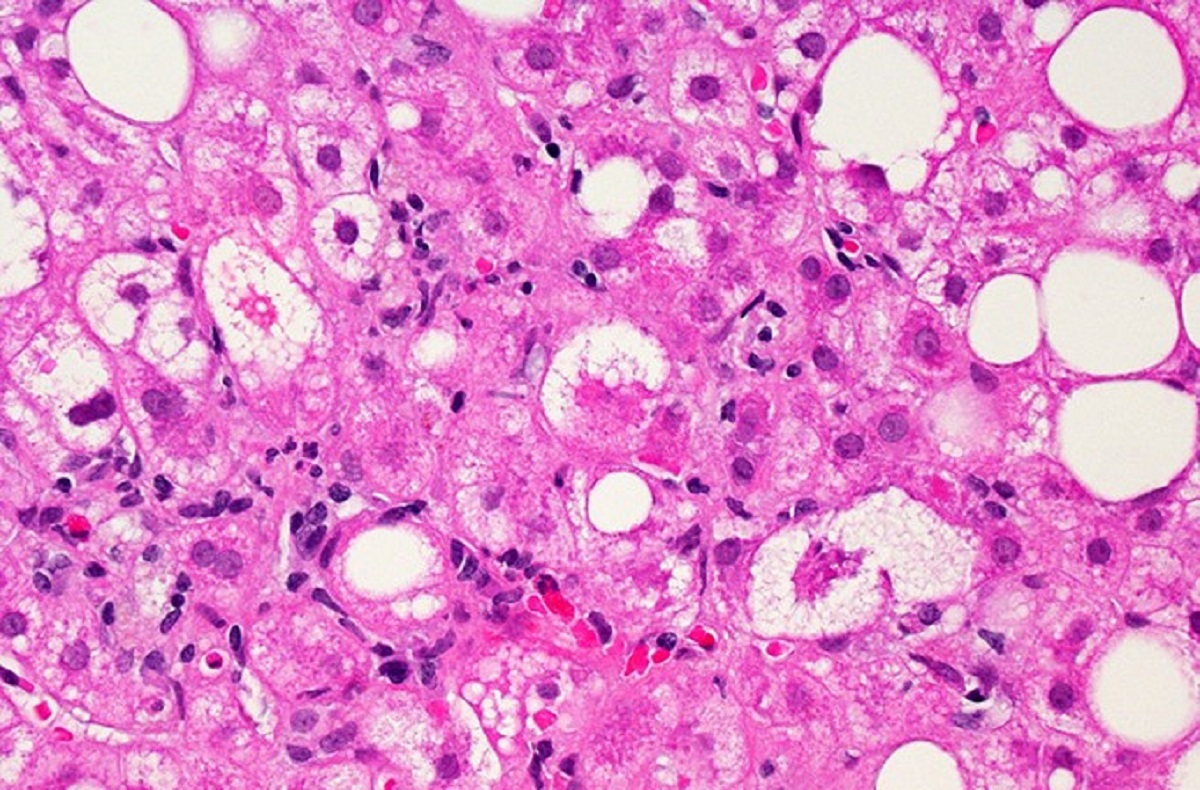
NASH is a fatty liver disease that leads to inflammation and damage of the organ. The disease, which can lead to liver failure, has no FDA-approved therapies. Intercept’s drug, obeticholic acid (OCA), is an analog of a naturally occurring human bile acid. It’s designed to bind to and activate a receptor expressed in the liver and intestine that regulates inflammatory, fibrotic, and metabolic pathways.
The Phase 3 clinical trial, named REGENERATE, has two main goals. One is showing a reduction in liver scarring, also called fibrosis. The other is showing resolution of NASH without worsening of liver fibrosis. The company had previously reached agreement with the FDA that meeting just one of the two goals would be enough for the Phase 3 test to be a success.
According to interim results for 931 patients released Thursday, treatment with the high dose of OCA led to 22.4% of patients showing improvement at 18 months compared to 9.6% of patients given a placebo, as assessed by a liver biopsy. On the study’s second main goal, a numerically greater number of participants treated with OCA met the goal but not enough to distance the therapy from the placebo group.
Both results are consistent with the original analysis in 2020 that led the FDA to reject the Intercept drug. The Morristown, New Jersey-based company Thursday said that the same subjects and the same liver biopsies were read in the new interim analysis. The difference this time is that latest results were produced by using a new methodology for liver biopsy analysis that the FDA is requiring to support a new drug application. In this “consensus read methodology,” panels comprised of three board-certified pathologists demonstrated the accuracy and reproducibility of liver biopsy assessments. The new interim analysis reassessed the liver biopsies at baseline and at 18 months.
“Achieving a statistically significant histology endpoint has proven to be an extremely high bar in clinical trials for NASH,” Jerry Durso, Intercept’s president and CEO, said in a prepared statement. “We are thrilled that OCA has demonstrated consistent improvement in fibrosis based on a second methodology and we now have two positive, statistically significant results for this primary endpoint from our pivotal REGENERATE trial.”
A blinded group of independent experts reviewed liver, heart and kidney safety events, a measure of safety that Intercept said was specifically requested by the FDA. The company said that OCA has been evaluated in 2,477 people who have taken at least one dose of the once-daily oral drug. Nearly 1,000 study participants have been on the drug for nearly four years.
Intercept said that the preliminary analysis through four years of treatment showed a numerically higher number of adjudicated liver safety events for the high dose of obeticholic acid. Most of these events were classified as mild. For adjudicated cardiovascular and kidney injury, Intercept said that the frequency of such events was low and balanced across treatment groups.
The most common side effect was severe itching, or pruritus, which was reported by 24% of those in the placebo arm and by 55% of patients who received the high dose of the Intercept drug. Pruritus was the most common reason cited for patients discontinuing treatment. Severe pruritus is also a side effect of the drug in the treatment of the primary biliary cholangitis, a disease of the bile ducts. For that approved indication, the Intercept drug is marketed under the name Ocaliva.
NASH’s growing prevalence along with the dearth of approved therapies for the disease has sparked numerous R&D efforts. The FDA’s rejection of Intercept’s drug two years ago is one of several high-profile setbacks for the field. Genfit’s NASH drug candidate, elafibranor, failed a pivotal study in 2020, which led the company to turn its efforts to a rarer liver disorder. Late last year, Genfit sold rights to the drug to Ipsen for €120 million up front.
Other R&D efforts are continuing. A partnership between Gilead Sciences and Novo Nordisk is testing various drug combinations in NASH. GSK jumped into the mix of NASH contenders last year, paying $120 million to acquire rights to an early-stage NASH drug candidate from Arrowhead Pharmaceuticals. Other NASH drug contenders include Madrigal Pharmaceuticals, Viking Therapeutics, Akero Therapeutics, and 89bio.
Public domain image by the Flickr user NIH Image Gallery














