Genentech starts phase 2 trial to optimize delivery of eye disease cell therapy
Fierce Pharma
NOVEMBER 28, 2022
Genentech starts phase 2 trial to optimize delivery of eye disease cell therapy. ntaylor. Mon, 11/28/2022 - 10:11.

Fierce Pharma
NOVEMBER 28, 2022
Genentech starts phase 2 trial to optimize delivery of eye disease cell therapy. ntaylor. Mon, 11/28/2022 - 10:11.

PharmaVoice
NOVEMBER 28, 2022
After nearly five years, the homicide case involving pharma billionaires continues to perplex investigators.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
NOVEMBER 28, 2022
Sanofi moves into swanky new Paris HQ designed around hybrid work and sustainability. fkansteiner. Mon, 11/28/2022 - 08:20.
MedCity News
NOVEMBER 28, 2022
Cincor Pharma drug baxdrostat failed its Phase 2 test, falling short of the goal of reducing blood pressure in patients with uncontrolled hypertension. But results in a subgroup of patients showed double-digit declines, which the company said could inform the design of a Phase 3 clinical trial.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
NOVEMBER 28, 2022
Biosimilars on the Cusp of Explosive Growth. jpiatt. Mon, 11/28/2022 - 12:16.
Pharmaceutical Technology
NOVEMBER 28, 2022
C4X Discovery (C4XD) and AstraZeneca have entered an exclusive global licensing agreement worth up to $402m to develop oral therapy to treat inflammatory and respiratory ailments. The deal has been signed to develop the C4X NRF2 Activator programme for these ailments. Under the agreement, AstraZeneca will be responsible for the development and marketing of oral therapy for inflammatory and respiratory diseases with a key focus on chronic obstructive pulmonary disease (COPD).
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

MedCity News
NOVEMBER 28, 2022
Healthcare has historically trailed other industries when it comes to the adoption of new technology. But providers that fail to get serious about bucking this trend are putting their businesses at risk for closure, according to John Barto, Microsoft’s chief digital transformation officer. Many clinicians end up leaving behind institutions that make them feel like they are stuck with archaic workflows and data management systems, he said.

Fierce Pharma
NOVEMBER 28, 2022
Spying new horizons, Xeris starts work on subcutaneous version of rare disease drug. ntaylor. Mon, 11/28/2022 - 10:15.

pharmaphorum
NOVEMBER 28, 2022
With COP27 concluding in November, Ben Hargreaves takes a look at what efforts pharma companies are making to limit their environmental impact. The past eight years are set to be the eight warmest on record. The rate of sea level rise has doubled since 1993; the past two and half years alone represent 10% of the overall rise in sea level since records began 30 years ago.

Fierce Pharma
NOVEMBER 28, 2022
Kyowa Kirin offloads mature drugs to Grünenthal amid 'challenging business environment'. aliu. Mon, 11/28/2022 - 10:22.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

pharmaphorum
NOVEMBER 28, 2022
According to a report by the Office of Health Economics (OHE), commissioned by the Association of the British Pharmaceutical Industry (ABPI), immediate action must be taken by governments, health systems, and companies to secure the era of green pharmaceuticals. ‘Supporting the era of green pharmaceuticals in the UK’ highlights that, while Britain can play a leading role in the sustainability agenda for pharmaceuticals, action must also be taken on a global scale to ensure impact.

European Pharmaceutical Review
NOVEMBER 28, 2022
FACILITATE , (FrAmework for ClInicaL trIal participants’ daTA reutilisation for a fully Transparent and Ethical ecosystem) is a patient-driven legal framework that intends to help return clinical trial data to study participants, ready for re-use in further research or healthcare practice. Part of a four-year project, aided by 27 partners from both EU and non-EU Member States, the framework will help develop technological solutions to support the sharing and re-use of the data.

pharmaphorum
NOVEMBER 28, 2022
Anthropy 2022 brought climate change to industry in Cornwall in (a very wet and windy) early November, settling cosily into the truly eco-minded environs of the Eden Project and firing up fervent and necessary conversations on sustainability, accessibility, and action plans. Amidst all this, Adelphi Group’s CEO Stuart Cooper found a moment to sit with pharmaphorum web editor Nicole Raleigh and discuss just how exciting it was to bring to vibrant and discursive life the ethos of Anthropy – a unit

MedCity News
NOVEMBER 28, 2022
Healthcare organizations need to have clear and specific goals in order to make real change in reducing health disparities, executives from SCAN Health Plan and CVS Health said. The insurer is tackling medication adherence in Hispanic members and flu vaccination rates in Black members, while the retailer is taking on women’s health, heart health and mental health.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
Legacy MEDSearch
NOVEMBER 28, 2022
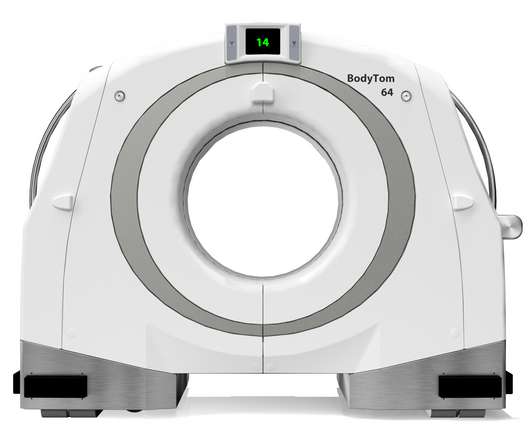
NeuroLogica Corp., a subsidiary of Samsung Electronics Co. Ltd., announced that its head-to-toe trauma imaging solution, the BodyTom® 64 Point-of-Care Mobile Computed Tomography (CT) Scanner, has received 510(k) clearance from the U.S. Food and Drug Administration for commercial use in the United States. “We’re thrilled to build off our expertise and elevate point-of-care imaging with our BodyTom 64, which can transform any room in a hospital into an advanced imaging suite,” said Jason Koshnitsk

Pharmaceutical Technology
NOVEMBER 28, 2022
The UK Government has announced funds worth $24.17m (£20m) to conduct research for developing new obesity therapies and technologies. These therapies and digital tools have been demonstrated to aid people in shedding 20% of their weight. Every year, obesity costs a staggering $7.2bn (£6bn) to the UK National Health Service (NHS), and by 2050, the figure is expected to increase to more than $11.7bn (£9.7bn) per year.

MedCity News
NOVEMBER 28, 2022
Fewer than half of female workers receive a paid parental leave benefit from their employer, including maternity leave (43%) and family and medical leave to care for a sick family member (44%), according to KFF.

European Pharmaceutical Review
NOVEMBER 28, 2022
Fast Track designation (FTD) has been granted for REM-001 therapy to treat unresectable cutaneous metastatic breast cancer (CMBC) by the US Food and Drug Administration (FDA). If marketed, REM-001, produced by Kintara Therapeutics, could be a potential therapy for advanced breast cancer patients who have limited treatment options. The drug was evaluated in four Phase II/III clinical trials in patients previously given chemotherapy and/or failed radiation therapy.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Pharma Pathway
NOVEMBER 28, 2022
Lee Pharma Limited- Openings for Warehouse/ QC/ R&D/ AR&D/ Maintenance/ Green Field Projects- Apply Now. Job Description. Lee Pharma Ltd, An ISO 9001: 2000 & WHO GMP Certified, One of the Best and Largest Manufacturers of Bulk Drugs & Intermediates in India. It is a Joint Venture with a renowed MNC having strong focus on Exports. We believe in Quality, result of persistent efforts.

European Pharmaceutical Review
NOVEMBER 28, 2022
Janssen’s SPRAVATO ® (esketamine nasal spray [NS]) helped more participants with treatment-resistant major depressive disorder (TRD) remain remission and relapse free compared with quetiapine extended-release (XR), both in combination with a continuing selective serotonin reuptake inhibitor (SSRI) or serotonin and norepinephrine reuptake inhibitor (SNRI), in a Phase III trial ( 04338321 ).

Pharma Pathway
NOVEMBER 28, 2022
Aurobindo Pharma-Openings for M.Sc in Regulatory Affairs Department- Apply Now. Company Profile: Aurobindo Pharma Ltd’ (APL). APL is a growing India multinational pharmaceutical manufacturing firm with turnover of over US$2.8 Billion revenues for 2018-19, with presence in more than 34 countries fronted presence with products exported to 155 nations.
MedCity News
NOVEMBER 28, 2022
In rejecting Spectrum Pharmaceuticals drug poziotinib, the FDA said the biotech needs to generate more data from another clinical trial. Instead, Spectrum is turning the company’s focus to commercializing its recently approved product for treating a common cancer complication.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

PharmaVoice
NOVEMBER 28, 2022
Psychedelic drug trials pose a number of challenges, but researchers can take these steps to boost patient safety.
Pharma Pathway
NOVEMBER 28, 2022
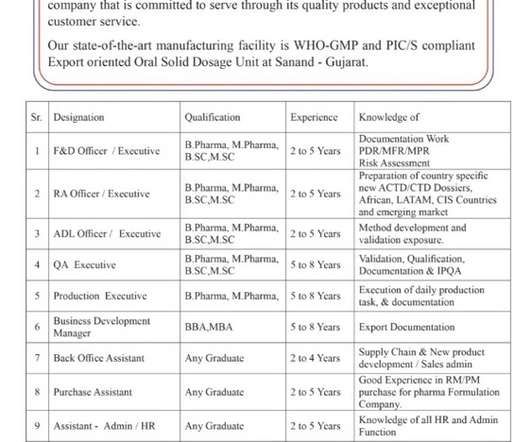
HOF Pharmaceuticals Limited-Openings for Production/ QA/ Regulatory Affairs/ F&D/ ADL/ Business Development/ Purchase/ Admin / HR/ Back Office- Apply Now. Job Description. Openings for Production/ QA/ Regulatory Affairs/ F&D/ ADL/ Business Development/ Purchase/ Admin / HR/ Back Office @ HOF Pharmaceuticals Limited. Departments: Production/ QA/ Regulatory Affairs/ F&D/ ADL/ Business Development/ Purchase/ Admin / HR/ Back Office.

PharmExec
NOVEMBER 28, 2022
Pfizer CEO Albert Bourla, Ph.D., is in trouble for "misleading" and "overly promotional" claims he made during a BBC interview late last year about their COVID-19 vaccine for younger kids, which was not approved at the time.

Pharma Pathway
NOVEMBER 28, 2022
Emcure Pharmaceuticals- Openings for ITI Fitter/ Electrical Technician/ Electrical Engineer/ Instrument Engineer-Apply Now. Job Description. Emcure Pharmaceuticals, We are a fast-growing Indian Pharmaceutical company engaged in developing, manufacturing, and marketing a broad range of biopharmaceutical products globally. Emcure’s differentiated product portfolio lends and unparalleled competitive advantage establishing its presence in all major therapies in the domestic market.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Map My Customers
NOVEMBER 28, 2022
Just like in any other professional field, continuing education is important in sales. Markets, client bases, and trends are constantly changing, and the modern sales professional ought to do what they can to stay effective, and stay fired up about their work. This can come through sales seminars and conferences, going back to school, or other opportunities offered by your company.

PharmaTimes
NOVEMBER 28, 2022
C4XD will receive milestone payments worth up to $16m ahead of the first clinical trial

MedReps
NOVEMBER 28, 2022
Interviewing for a medical device sales job can be nerve-wracking. So, it helps to be as prepared as possible, dressing professionally for your interview, carrying along a copy or two of your resume and reference list, and of course, practicing your quick elevator pitch so you can introduce yourself quickly if needed. On top of these preparation methods, you can also spend some time reviewing the answers to popular interview questions.

PharmaTimes
NOVEMBER 28, 2022
Project involves 27 partners including patient associations, hospitals and universities

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content