RxBenefits, Tria Health form partnership for proactive diabetes care
MedCity News
JULY 14, 2022
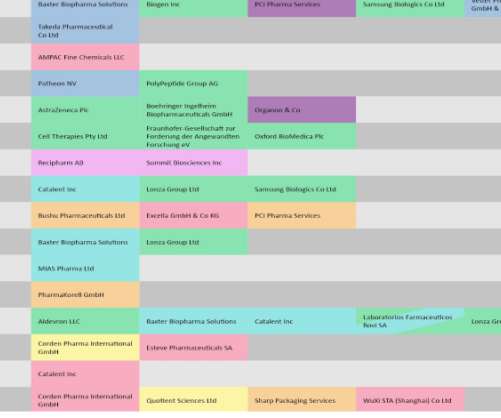
RxBenefits, a pharmacy benefits optimizer, and Tria Health, a chronic condition and medication management company, have launched a new partnership to improve diabetes care and lower healthcare costs for the condition through a proactive approach. .

































Let's personalize your content