Medical device and MedTech insights, news, tips and more
FDA Clears Dexcom’s First Over-the-Counter Continuous Glucose Monitor
March 7, 2024

On March 5, the U.S. Food and Drug Administration (FDA) announced the clearance of Dexcom’s device, marking it as the first continuous glucose monitor available over the counter. Following this announcement, Dexcom’s shares experienced a 2.2% increase in extended trading. Known as Stelo, this device is tailored for individuals aged 18 and older who do not require insulin, who are managing diabetes via oral medications or individuals without diabetes seeking to understand how diet and exercise affect blood sugar levels.
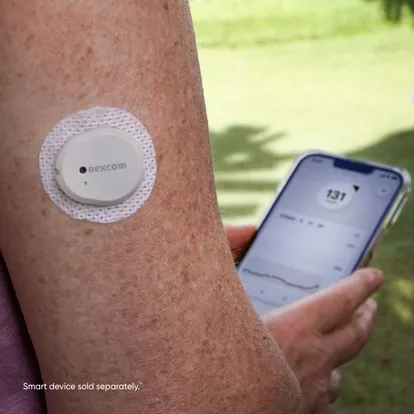
However, the FDA cautioned that Dexcom’s Stelo is not suitable for individuals experiencing problematic hypoglycemia or low blood sugar, as it has not been designed to alert users of this potentially hazardous condition. The device relies on a wearable sensor, which communicates with a smartphone application to continuously monitor blood glucose levels providing valuable insight into users’ blood sugar fluctuations.
Dexcom disclosed its plans to make Stelo available for purchase online without the need for a prescription, starting in summer 2024. The company aims to offer the device at a competitive price point, but further pricing details will be shared closer to the product’s release date. This move signifies a significant advancement in making continuous glucose monitoring technology more accessible to a broader population, facilitating better management of diabetes and promoting proactive health monitoring.
See Full Press Release at the Source: FDA Clears First Over-the-Counter Continuous Glucose Monitor
Press Release by: US Food and Drug Administration

Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 19 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
